Geoscience Reference
In-Depth Information
properties in particular is very important. A second reason
to be interested by clays comes from their very small par-
ticle size (typically smaller than 5
0.9
0.8
m) and the charged
nature of their crystalline planes (Figure 1.8). The small
size of the clay particles implies that they carry a huge
charge per unit pore volume of porous rocks. There
are at least two families of clay minerals depending on
whether the space between the clay crystals is open or
closed: on the one hand, kaolinite, chlorite, and illite
have no open interlayer porosity, while on the other
hand, smectite has an interlayer porosity strongly influ-
encing its swelling properties. Figure 1.8 shows that
the surface charge density of the clay particles has two
distinct origins: one is located essentially on the basal
planes that is mostly due to isomorphic substitutions in
the crystalline framework and is pH independent (this
charge is dominant for smectite). The second charge den-
sity is mostly located on the edge of the crystals due to
amphoteric (pH-dependent) active sites.
The small particle size of clay minerals implies in turn a
high specific surface area and a high cation exchange
capacity (CEC) by comparison with other minerals. The
specific surface area
S
sp
(in m
2
kg
−
1
) corresponds to the
amount of surface area divided by the mass of grains.
The CEC (in C kg
−
1
) corresponds to the amount of charge
that can be titrated on the mineral surface divided by the
mass of mineral. The ratio of the CEC by the specific sur-
face area corresponds to the effective charge density on
the mineral surface:
μ
0.7
0.6
Sample
pH = 9.5
#4
#3
0.5
9.2
#2
0.4
9.0
#1
0.3
C
1
=1.07±0.13
log
K
1
=
-6.73±0.11
0.2
log
K
3
=
-0.25±0.20
0.1
10
-4
10
-3
10
-2
10
-1
(mol l
-1
)
Salinity,
C
f
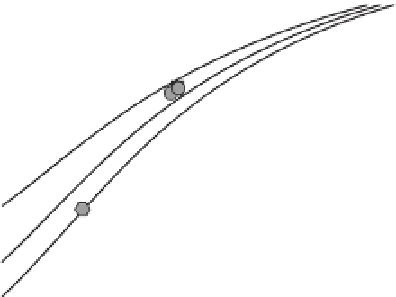
Figure 1.6
Partition coefficient versus the salinity of the free
electrolyte with the TLM parameters indicated on Figure 1.2 for
NaCl (pH = 9, 9.2, 9.5). The symbols correspond to the partition
coefficient determined from the complex conductivity data for
the seven experiments described in the main text. The data are
determined from spectral induced polarization measurements
(see Leroy et al., 2008). They show an increase of the partition
coefficient with the salinity and the pH in fair agreement with
the model.
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
10
-4
pH 10
pH 9
Q
0
=
CEC
S
sp
1 53
pH 8
pH 7
As shown in Figure 1.9, the charge per unit surface is
pretty constant for all clay minerals and comprised bet-
ween 1 and 3 elementary charges per nm
2
at near-neutral
pH values. Because part of the charge on the surface of the
clayminerals is pHdependent,Maes et al. (1979) proposed
for 3.9
pH 6
pH 5
10
-3
10
-2
10
-1
Salinity (mol l
-1
)
5.9 and formontmorillonite (a special type of
smectite) the following pH-dependent relationship for
the CEC: CEC (in meq g
−
1
) = 79.9 + 5.04 pH for monova-
lent cations and CEC (in meq g
−
1
) = 96.1 + 3.93 pH for
divalent cations.
A theory for the electrical double layer of clay minerals
is now introduced. This theory can be used to predict
the amount of charge on the mineral surface and in the
Stern layer or more directly the zeta potential. It can also
be used to predict a highly important parameter used in
≤
pH
≤
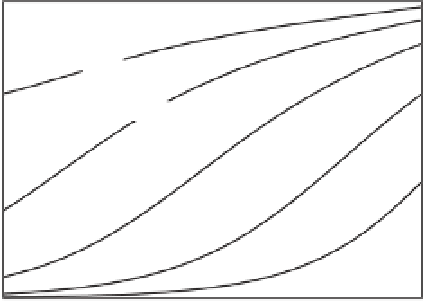
Figure 1.7
Determination of the partition coefficient though a
triple layer model for silica for different values of the pH and
salinity of NaCl solutions.
the diffuse layer, while at high salinity (>10
−
3
mol l
−
1
),
the counterions are mostly located in the Stern layer.
1.1.2 The case of clays
Clays are ubiquitous in nature, and as such, their influence
on electrical properties in general and the seismoelectric