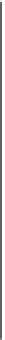Geoscience Reference
In-Depth Information
Table 1.1
Equilibrium constants for surface complexes at the
surface of a silica sand.
Gaudin and Fuerstenau (1955) NaCl
Li and de Bruyn (1966) NaCl
Watillon and de Backer (1981) KNO
3
+
Reactions
Equilibrium constants
Jaafar et al. (2009) NaCl
-140
>SiOH + H
+
>SiOH
2+
10
−
2.2
>SiO
−
+ H
+
10
−
6.2
>SiOH
+
+
-120
>SiO
−
+Na
+
>SiO
−
Na
+
10
−
4.5
+
>SiOH +Cu
2+
>SiOCu
+
+ H
+
10
−
3.4
-100
2 >SiOH +Cu
2+
2(>SiO)
−
Cu
2+
+2H
+
10
−
8.8
+
>SiOH + SO
4
2
−
+ H
+
>SiSO
4
−
+ H
2
O
10
5.0
-80
+
From Sverjensky (2005).
-60
-40
Shear plane
OHP
-20
Model
XCu
+
X
-
Cu
2+
Cu
2+
Cu
2+
0
SO
2
-
4
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
10
0
10
1
Cu
2+
Electrolyte concentration (salinity mol l
-1
)
X
-
SO
2
4
Figure 1.3
Zeta potential
on the surface of a silica grain.
Comparison between the analytical model developed in the main
text (plain line, Eqs. 1.31
ζ
X
-
Cu
2+
Cu
2+
X
-
1.33) and experimental data from the
literature. These data are from Gaudin and Fuerstenau (1955),
Li and De Bruyn (1966), Watilllon and De Backer (1970), and
Jaafar et al. (2009). We use pH = 5.6 (pH of pure water in
equilibrium with the atmosphere),
K
(
−
)
=10
−
7.4
, and a density
of surface active site at the surface of silica of
-
SO
2
4
XCu
+
SO
2
4
X
-
XSO
4
X
-
XCu
+
SO
2
-
4
Cu
2+
Cu
2+
SO
2
-
4
0
S
= 7 sites nm
−
2
.
Note the high salinity values are not captured by the model.
Γ
Cu
2+
SO
2
4
Cu
2+
In addition, the analysis made earlier is correct only for
silica in contact with simple supporting electrolytes such
as NaCl or KCl with a weak sorption of the counterions.
As mentioned briefly previously, the composition of the
pore water can, however, strongly influence the value
and even the sign of the zeta potential. In the case of
strong sorptions, it is necessary to account for more intri-
cate complexation reactions on the surface of silica like
the one shown in Table 1.1 for copper. Figure 1.4 shows
the speciation of copper on the mineral surface forming
both monodentate and bidentate complexes. In the pres-
ence of such strong sorption phenomena, the zeta poten-
tial can reverse sign and drastically change in magnitude.
This is especially true in the case of the sorption of cations
of high valence (e.g., Al
3+
) directly on the mineral sur-
face. In such inner-sphere complex, the cation loses part
of the hydration layer. The charge density of the counter-
ions in the Stern layer can be high enough to overcome
the charge density on the surface of the mineral. In this
case, the charge of the diffuse layer and its associated zeta
o-plane
d-plane
Figure 1.4
Sketch of the electrical double layer showing the
speciation of copper and sulfate for a solution of copper sulfate in
contact with a silica surface. Sorption of copper on the mineral
surface (inner-sphere ligand) occurs as a monodentate complex
(immobile), while sorption in the Stern layer (outer-sphere
ligand) occurs as a (mobile) bidentate complex. This type of
sorption has a strong effect on the value of the zeta potential and
can, under given conditions, reverse the polarity of the zeta
potential on the surface of silica. The o-plane denotes themineral
surface, and the d-plane denotes the outer Helmholtz plane
(OHP) on which the zeta potential
ζ
is considered.
potential have a reversed polarity, at a given pH, with
respect to what is normal for a simple supporting binary
electrolyte like NaCl or KCl. Electrokinetic phenomena
like the seismoelectric effect are very sensitive to these
types of chemical changes because they are directly con-
trolled by the properties of the electrical double layer and
by the zeta potential.