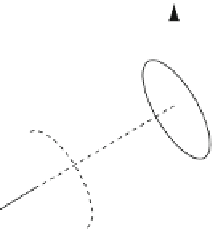Chemistry Reference
In-Depth Information
z = z'
z'
z'
z
θ
1
θ
2
x
z
x
x
y
y
y
Fig. 2 Variation of alignment using stretched polyacrylamide gels (SAG) and bacteriophage
Pf
1,
which has been embedded and aligned (along
z
0
) at different angles relative to the long axis (
z
)ofthe
sample. The gels were cast in an approximately ellipsoidal (squashed cylinder) geometry, with
dimensions of 5, 7, and 10 mm along the
x
,
y
,and
z
axes, respectively. The gels were then dried,
rehydrated, and stretched to fit within a 4.2 mm i.d. NMR tube. (Reprinted with permission from [
31
])
3.5 Conservative Mutation
The surface charge of
Pf
1 phage is heavily negative and proteins will be aligned
according to its surface electrostatic potentials [
40
]. Yao and Bax [
30
] carefully
modified the surface charge distribution of the 6-kDa protein GB3 by either conserva-
tively mutating one or two residues at each time, e.g., K to E, or keeping the histidine-
tag at terminus of the native GB3. The backbone structure was later found to be
unperturbed in those modified proteins. A total of six mutant proteins were found to
align quite differently with respect to the
B
0
field and five singular valueswere obtained
(Fig.
3
). With this well-defined system, the amplitude and direction of RDC bond
dynamics up to the millisecond (ms) were unambiguously obtained and compared to
nanosecond to picosecond (ns-ps) dynamics from spin relaxation measurements
(Fig.
4
)[
41
]. Interestingly, Yao et al. found both RDC and relaxation measurements
showed the same amount of flexibility for residues in regular secondary structures.
Bond vectors within loop regions, however, were shown by RDC to have larger
amplitude of motions compared to what were suggested from the relaxation data [
41
].
3.6 RDC/RCSA Accuracy Improvement
In addition to RDC, protein alignment creates slight chemical shift changes for the
aligned sample relative to the isotropic sample. The chemical shift difference,
named residual chemical shift anisotropy (RCSA), comes from the projection of
the chemical shift tensor, which is not averaged to zero, onto the alignment tensor.
RCSA is also a long range structural restraint, providing orientation dependences