Chemistry Reference
In-Depth Information
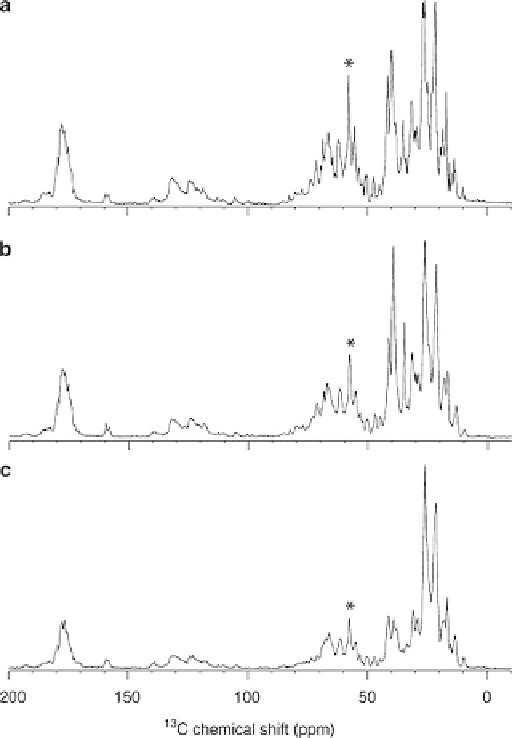
Fig. 1 1D
13
C NMR spectra (800 MHz for
1
H) of CN-bR (a), HDCN-bR (b), and DCN-bR
(c), fully hydrated in sodium citrate buffer and collected under very similar conditions. Adapted
from [
119
] with permission from Elsevier B.V
13
C-DQ/SQ correlation experiment on the fully hydrated [
13
C
6
,
15
N]-Leu-BR
sample using a Bruker AV-III 600 MHz wide-bore spectrometer with an MAS
rate of 8 kHz (Fig. 2a). The sample activity is carefully assessed through detection
of the M state and the proton pumping cycle at 412nm and 456nm by optical
dynamic spectroscopy, respectively. It is clear that the resolved narrow spectral
linewidths are attributed to the predominant distribution of the Leu residues on the
helical segments with similar local environments, and the up-field shifted peak is
attributed to the Leu residue located at the loop region. This small shift clear
indicates a homogenous sample condition. The expressed protein is fully function-
ing as confirmed by capture of the M state signal and the proton pumping cycle
signal shown in Fig 2b and c.