Chemistry Reference
In-Depth Information
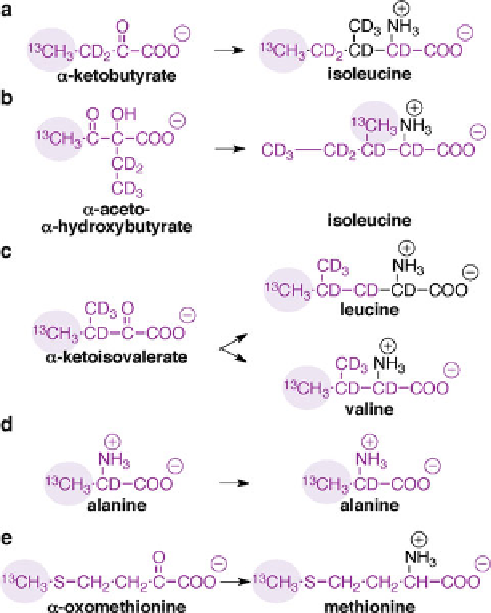
Fig. 5 Metabolic precursors added to expression media prior to induction of expression for the
selective incorporation of
13
C and
1
H into methyl groups of
12
C,
2
H-labeled (a) Ile (C
d
1-labeled),
2-labeled), (c) Leu and Val, (d) Ala, and (e)
13
C,
1
H-Met. The parts of the amino acid that
originate from each precursor are shown in
purple
, with the
13
C,
1
H-labeled methyl group
highlighted. In all cases the expression mediummust include
13
C,
2
H-labeled glucose as the carbon
source. Selective methyl labeling of Ala also requires media supplementation with deuterated
succinate,
(b) Ile (C
g
-ketoisovalerate and isoleucine to suppress isotope scrambling. In this procedure
glucose and glycerol together are used as the carbon sources prior to addition of methyl labeling
agents [
263
]
a
NOEs involving methyl protons yielded a fourfold increase in the number of
distance restraints over
1
H
N
-
1
H
N
NOEs alone [
71
], and a twofold increase in
NOE restraints for the 210 residue KpOmpA
-barrel [
70
]. Smaller gains were
obtained for the 283 residue VDAC-1, with 324 methyl-associated NOEs adding to
the 288 amide proton NOEs [
73
]. However, in all these cases, the overall fold of the
b
b
-barrel is already well-defined by NOEs between amide protons, and hence the
impact of methyl NOEs on structure quality tends to be modest, albeit significant,
for these folds (e.g., OmpX NOEs from methyls decreased the backbone rmsd to the
mean from 2.13
˚
to 1.42
˚
,[
71
]).
While the utility of methyl protonation for structure determination of
b
-barrel
folds has been demonstrated,
the full benefits of this strategy for structure