Chemistry Reference
In-Depth Information
3.2 Bicelles
Avoiding some of the potential problems associated with micellar detergents, small
isotropic bicelles have increasingly been used for solution NMR of membrane
proteins (reviewed in [
172
-
174
]). These are formed by mixtures of up to a fivefold
molar excess of short-chain lipid (e.g., C
6
-DHPC) or detergent (e.g., CHAPS) over
long-chain lipid (e.g., DMPC, DOPC) [
175
,
176
]. The composition of a bicelle is
most accurately described by
q
, the molar ratio of lipid and
bicellar
detergent
(i.e., [detergent]
bicelle
¼
[detergent]
total
- cmc) [
112
], which for small isotropic
bicelles is typically in the range of 0.25-0.5. It has been shown that bicelles with
this range of
q
values have a disk-shaped morphology, containing a distinct lipid
bilayer phase and edges coated by a more mobile detergent phase (Fig.
3
)[
177
-
179
].
The size of the protein-free small bicelle depends on
q
, and can be comparable to the
size of commonly used detergent micelles (e.g., ~22 kDa for
q
¼
0.15 [
180
]).
However, spectra obtained for proteins reconstituted in bicelles have generally
shown broader peaks than those for the same sample in a micelle [
40
,
137
,
181
,
182
], with the larger complex size for the bicelle-protein complex contributing to
this broadening. Yet in some cases only a bicelle environment could uniquely confer
a functionally folded, spectrally homogeneous sample (e.g., the small multidrug
resistance pump (Smr) [
183
]). A bicelle formulation mimicking physiological
membrane compositions was also found to be instrumental for structure determina-
tion of the weakly interacting integrin
a
IIb
b
3 TM-heterodimer [
184
], a complex that
was not supported by DPC micelles [
185
].
In contrast with micelles, the introduction of proteins into bicelles may require
an additional optimization step, since there are a few different approaches that can
be used [
186
,
187
]. However, most membrane proteins that have been reconstituted
into small isotropic bicelles for solution NMR could be prepared in solvent-free
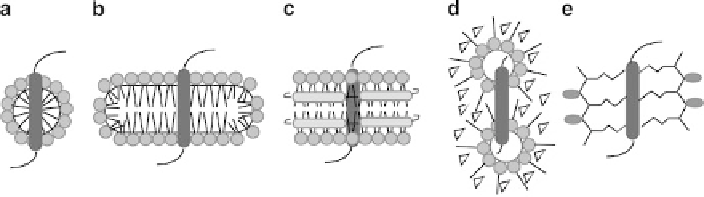
Fig. 3 Schematic diagram showing the general structure of various membrane mimetic systems
used for solution NMR studies of membrane proteins. One TM segment is shown embedded in
(a) a micelle, (b) a bicelle, (c) a nanodisc, (d) reverse micelles, and (e) amphipols. Polar detergent
or lipid headgroups are represented by
spheres
, with hydrophobic acyl chains as
straight lines
.
The MSPs surrounding the periphery of the nanodisc are shown as
gray rods
, and co-surfactants or
co-solvents that stabilize reverse micelles are shown as
triangles
. Two amphipols are shown
surrounding the TM segment in (e), with the polar blocks (
gray
) connected to hydrophobic blocks
(
lines
) that interact with the protein