Chemistry Reference
In-Depth Information
Aside from mixtures that alter the charge of micelle surfaces, it is also possible
to choose detergent combinations that alter the mean size of the micelle hydropho-
bic core. Analysis of small angle X-ray scattering data showed that the smaller
hydrophobic core in short-chain detergent micelles can be increased by the addition
of longer-chain detergents [
155
,
156
]. A linear relationship between micelle size
and detergent long- to short-chain molar ratios was observed, suggesting that
micelle dimensions could be tuned in a straightforward manner to match the size
of a protein hydrophobic domain. For a model 2-TM helix system, detergent
mixtures that optimized hydrophobic matching between micelle and protein gave
rise to the highest quality spectra, and also promoted more compact protein
structures [
155
]. Although the applicability of these trends for other membrane
proteins remains to be established, this study has identified an additional parameter
that can be explored when optimizing protein-detergent complexes for solution
NMR.
3.1.6 Potential Drawbacks to the Use of Micellar Membrane-Mimetics
While most solution NMR structures of larger membrane proteins have been
elucidated in non-SDS detergent micelles, there are some caveats to keep in mind
whenever any detergent is used to study membrane protein structure (Fig.
2
). For
example, with most NMR-friendly detergents having high cmcs, there is a signifi-
cant concentration of monomeric detergent in the solvent that can potentially bind to
solvent-exposed regions of the protein that do not normally interact with lipids.
X-ray crystal structures have provided some examples of this, with detergent being
found in the active site cavity of the
b
-barrel PagP [
157
], and inside the M2 channel
[
158
]. For smaller membrane proteins this is particularly significant since there are
fewer intramolecular interactions that stabilize the protein fold compared to the
relatively large number of detergent-protein interactions. This larger proportion of
residues that are exposed to solvent detergent
increases the potential
for
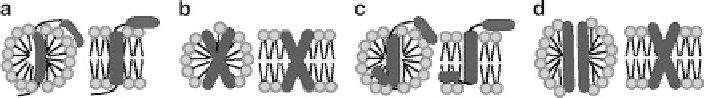
Fig. 2 Schematic representation of potential changes in integral membrane protein structure that
could be imposed by a micellar environment (left hand side of each panel), compared to the native
structure in bilayers (
right
). Possible distortions include; (a) micelle-induced curvature in the TM
helix or amphipathic helix; (b) monomeric detergent molecules bound to a solvent-exposed
region, in this case an aqueous cavity close to the micelle surface; (c) altered relative orientations
of amphipathic vs TM helices; (d) loss of tilt relative to other TM segments. In this scenario
hydrophobic mismatch between the TM helix and micelle are minimized by distortions in micelle
structure that allow hydrophobic protein surfaces to remain in the hydrophobic phase. In the
bilayer environment hydrophobic mismatch induces tilt, favoring a non-zero inter-helical crossing
angles