Biology Reference
In-Depth Information
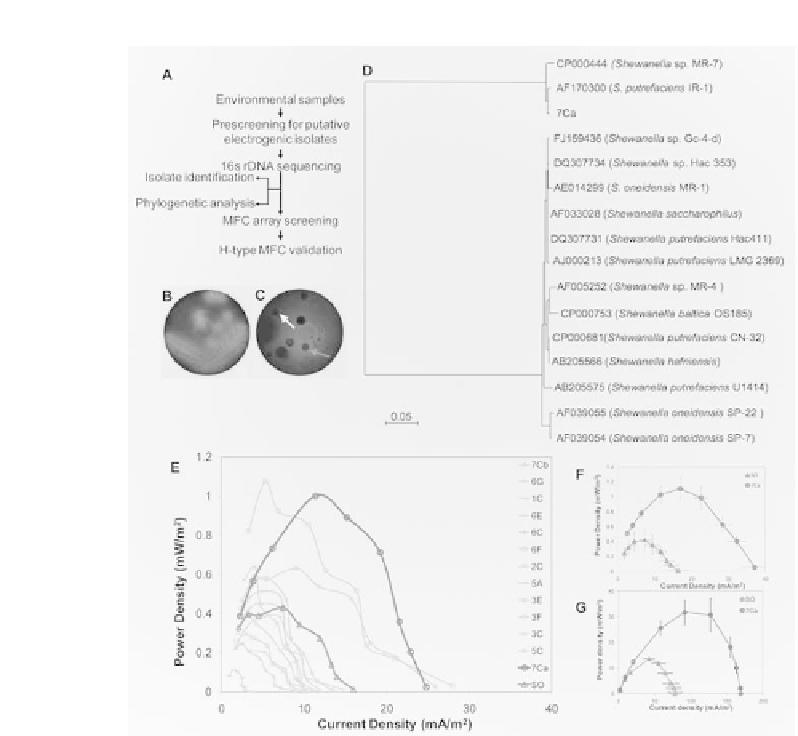
Figure 4.
Environmental sample screening with the MFC array. (A) A schematic representation of the
screening process for the environmental microbes with enhanced electricity generation capacities.
(B) and (C) Electrochemically active microbes cause discoloration of an azo dye, reactive black 5 in
the nutrient agar screening plate. (B)
S. oneidensis
MR-1 was used as the control; (C) A representative
plate with a putative and non-putative microbe isolate indicated by arrow. (D) Phylogenetic tree
based on 16S rDNA sequences indicating the relationship of various
Shewanella
species. (E)-(G)
Screening of environmental isolates using the 24-well MFC array. (E) Screening of 13 environmental
isolates with
S. oneidensis
MR-1 as the positive control (SO) using two 24-well MFC arrays in
parallel. The average power density of two replicates was shown for each isolate. (F) The power
density of 7Ca compared to the
S. oneidensis
MR-1 reference strain (n = 8). (G) Validation of current
generation by 7Ca and
S. oneidensis
MR-1 in conventional MFCs (n = 4).
We have demonstrated that a microfabricated MFC array system can be exploited
to rapidly screen and characterize microbial electrochemical activity. The universal
design of our system has several attractive features. First, the microbe culture chamber
was easy to assemble and reusable. The PDMS and electrode could be used at least
ten times without degradation and the acryl anode chamber could be used more than
ten times with proper cleaning. Therefore, the device has the potential for widespread
adoption. Second, because the individual MFC chambers in the array hold a small vol-
ume, 380-fold fewer reagents are required than conventional MFC devices. Third, the