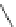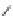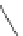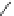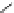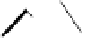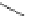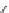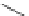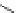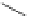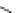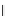Biology Reference
In-Depth Information
OH
O
O
O
O
HO
HO
HO
HO
HO
OH
+
NH
2
O
OH
OH
OH
HO
HO
O
HO
O
HO
O
P
O
2
O
Glc
4
GlcN-1-P
phosphorylase
OH
O
O
O
HO
HO
OH
+ inorganic phosphate
OH
NH
2
OH
HO
HO
O
HO
O
HO
3
α
-glucosaminyl-(1
�
4)-Glc
4
Figure 3.14
Phosphorylase-catalyzed
α
-glucosaminylation of Glc
using
4
GlcN-1-P.
-glucosaminylation was perfor-
med using GlcN-1-P as a glycosyl donor and Glc
The phosphorylase-catalyzed
α
as a glycosyl acceptor
(Fig. 3.13). After the reaction mixture was lyophilized,
4
-acetylation
was carried out using acetic anhydride, and the transfer of a GlcN
unit to the primer was evaluated by MALDI-TOF MS measurement.
Because the difference in the molecular masses of the anhydroglucose
and anhydroglucosamine units was only 1, which could be made
larger by the
N
-acetylation of the latter unit, the measurement was
performed on the
N
N
-acetylated material. In the MALDI-TOF MS
spectrum of the
-acetylated crude products, a significant peak
corresponding to the mass of a pentasaccharide containing one
GlcNAc unit was observed. This data indicated that one GlcN residue
transferred from GlcN-1-P to Glc
N
by the phosphorylase-catalyzed
4
α
-glucosaminylation. To confirm the presence of the GlcN unit at
a nonreducing end of the produced oligosaccharide, the treatment
of the
-acetylated crude products with GA was performed. In
the MALDI-TOF MS spectrum of the treated product, the peak
assigned to the molecular mass of the
N
N
-acetylgucosaminylated Glc
4
remained intact, supporting that the GlcNAc unit was positioned at
the nonreducing end. If the transfer of one GlcN residue from Glc-
1-P to Glc
proceeded once, further glucosaminylation was probably
suppressed because the glucosaminylated Glc
4
was not recognized as
the acceptor by phosphorylase. The main product was isolated from
4