Biology Reference
In-Depth Information
in the XRD patterns of the complexes including fatty acids and
PTHF, supported the longer diameter of the inclusion complexes.
These patterns were similar to those of the product obtained by
the vine-twining polymerization using the guest PLLA as described
earlier (Fig. 8.6d), indicating the longer diameter of the amylose-
PLLA inclusion complex compared with that of the amylose-PTHF
inclusion complex, owing to a bulky structure of PLLA due to the
branched methyl groups.
1
of the product obtained from
PLLA showed the signals owing to not only amylose but also PLLA,
in spite of washing with chloroform as the good solvent for PLLA,
indicating that the PLLA was included in the cavity of amylose.
To investigate the effect of the chirality in PLAs on the inclusion
of amylose, the vine-twining polymerization was performed using
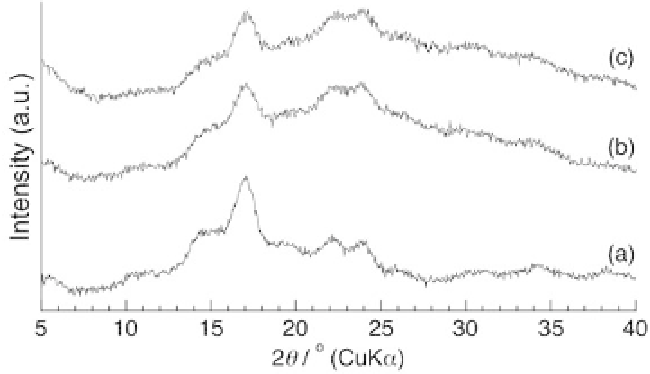
PDLAs (Fig. 8.5b) and PDLLA (Fig. 8.5c) as guests. Consequently, the
XRD patterns of the products showed only the diffraction peaks due
to amylose (Fig. 8.7) and the
The
H NMR spectrum in DMSO-
d
6
1
H NMR spectra did not show the signals
due to PLAs, indicating no formation of the corresponding inclusion
complexes. These results indicate that amylose perfectly recognized
the chirality in PLAs on the formation of inclusion complexes in
vine-twining polymerization. The modeling calculations supported
the amylose's chiral recognition in favor of PLLA and proposed
the atomistic details of the inclusion complex, which involved the
preferred orientation of the constituent molecular chains with
respect to their fiber axis.
Figure 8.7
XRD patterns of amylose (a) and the products obtained by
vine-twining polymerization in the presence of PDLA (b) and
PDLLA (c).