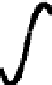Biology Reference
In-Depth Information
8.1.3
Amylose Recognizes Chirality in Poly(lactide)s
Importance of chirality has often appeared in nature because in most
cases only one of the enantiomers in the chiral biomolecule exhibits
in vivo
functions in biological systems. For example, in the case of
carbohydrates, their
d
-forms primarily exist in nature and show
each specific biological function. Besides such chiral biomolecules,
synthetic optically active polymers have also attracted much attention
because of various applications such as asymmetric syntheses,
chiral adsorbents for separation of racemates in HPLC, and liquid
crystals [5]. On the basis of the importance of a sole enantiomer in
the aforementioned chiral and optical active functions, a number
of chiral recognitions and separations have been performed in the
studies on various host-guest systems.
Because amylose is a polysaccharide with left-handed helical
(chiral) conformation, it can be considered to be a good candidate
for the host molecule that shows ability of chiral recognition toward
the optically active polymers such as poly(lactide)s (PLAs) by means
of the selective formation of the inclusion complex. Therefore, the
recognizable inclusion of amylose toward the chirality in PLAs in the
vine-twining polymerization was investigated [6].
First, the vine-twining polymerization was performed by
phosphorylase-catalyzed enzymatic polymerization of Glc-1-P from
Glc
in the presence of PLLA (Fig. 8.5a). The precipitated product was
characterized by means of the XRD and
7
1
H NMR measurements.
CH
3
(a) Poly(
L
-lactide)
(b) Poly(
D
-lactide)
(c) Poly(
DL
-lactide)
(PLLA)
(PDLA)
(PDLLA)
O
O
n
Glc
7
Glc-1-P
Amylose
PLLA
Phosphorylase
"Amylose stereoselectively includes PLLA"
Figure 8.5
Vine-twining polymerization using phosphorylase in the
presence of PLAs.