Environmental Engineering Reference
In-Depth Information
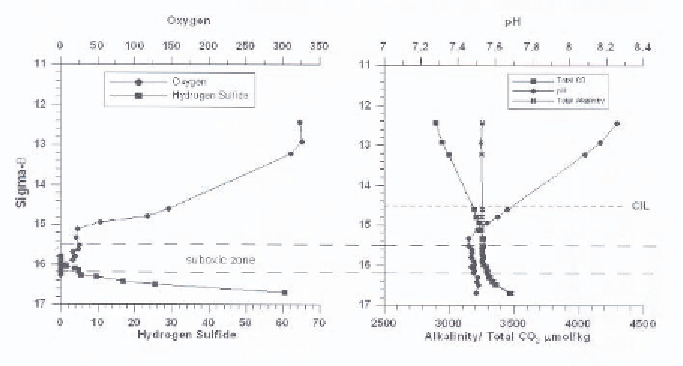
the oxic euphotic zone, total CO
2
increases with depth while alkalinity stays
approximately constant. This indicates a source of CO
2
, probably due to aerobic
respiration of organic matter. Both increase with depth below the suboxic zone
into the deep anoxic water. There must be significant production of alkalinity
within the Black Sea [12, 13, 16]. In the sulfide containing deep waters, total
CO
2
and alkalinity are dominated by sulfate reduction. Each mole of sulfide
produced is matched by two equivalents of alkalinity:
53
SO
4
2
−
=
106
HCO
3
−
+
+
106
CH
2
O
53
H
2
S
Goyet et al. [16] observed that dissolution of CaCO
3
caused both total CO
2
and alkalinity to increase faster than expected based on sequential oxidation of
Redfield organic matter by O
2
,NO
3
and SO
4
. Thus, total alkalinity needs to be
corrected for the minor bases: borate, ammonia, phosphate, silicate, bisulfide
and with Ca
2
+
representing CaCO
3
dissolution. Both calcite and aragonite are
undersaturated at depth in the Black Sea.
Figure 10.
Dissolved oxygen and hydrogen sulfide (left panel) and carbonate system â^ pH,
Alkalinity and Total CO
2
(right panel) - versus density from Knorr 2001; April 2001; leg 1, Stn
5 (Hiscock and Millero, unpublished data).
4.6 Manganese-Iron
The Black Sea has long been an important site for investigating the biogeo-
chemical cycling of Fe and Mn across redox boundaries. The studies of Mn
cycling by Spencer and Brewer [64] and Spencer et al. [65] are considered
classics. Typical profiles for dissolved and particulate manganese (together