Agriculture Reference
In-Depth Information
a few points to as much as 10 to 12. Some isotherms represent a concentration
range of three to four orders of magnitude (10
-3
-10
+2
mg/L) and some rely on
one single concentration. Data analysis is simple, where the amount of solute
adsorbed by the soil matrix represents the difference in concentration of the
final and initial solution.
This one-step method measures adsorption at one equilibration time
only. Thus, the method implicitly assumes that kinetics is not dominant and
equilibrium is attained during the measurement period. Moreover, this method
does not address the question of release or desorption following adsorption.
2.3.2 Kinetic Batch Reactor
This is perhaps the oldest method for measuring the rate of reactions under
controlled conditions. The method is similar in principle to that described
above except that it allows for repeated measurements at various times as
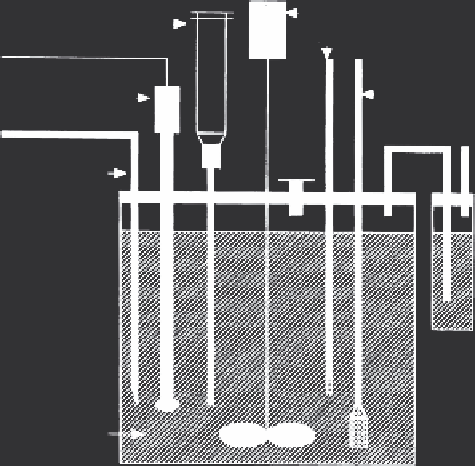
desired to quantify solute adsorption kinetics. As illustrated in Figure 2.16,
Batch Reactor
Stirrer
Syringe sampler
ermometer
Inert Gas
Dispersion
tube
Combination
pH Electrode
Acid or base
addition
Port
Gas
trap
Suspension
FIGURE 2.16
A typical batch reactor configuration. pH is controlled by a combination pH electrode and
automatic burette connected to an auto titrator. A syringe sampler allows for removal of a
subsample of suspension, an addition port permits injection of solute, an inert gas is bubbled
through the suspension by means of a gas dispersion tube, and the system is vented through
a gas trap. A thermometer allows for temperature monitoring, and the suspension is mixed
with an overhead stirrer. (From H. M. Selim and Amacher, M. C. 1997.
Reactivity and Transport
of Heavy Metals in Soils
. Boca Raton, FL: CRC Press. With permission.)
Search WWH ::

Custom Search