Agriculture Reference
In-Depth Information
Linear
200
50
150
40
Equil
30
100
20
50
10 hr
0
0
100
200
300
400 00
600
Adsorb
Desorb
150
50 hr
100
10 hr
50
0
0
100
200
300
400 00
600
Solution Concentration (μg/g)
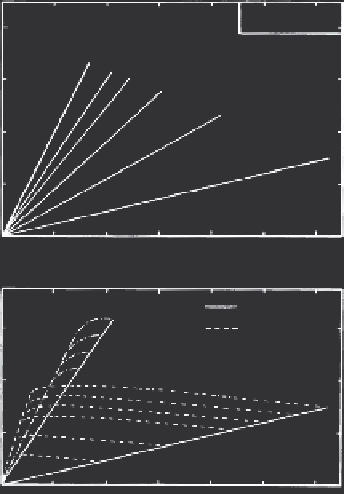
FIGURE 2.14
Simulated adsorption-desorption isotherms using a linear kinetic retention model. Desorption
was initiated after 10 and 50 hours for each successive sorption step.
well as desorption were carried out for times sufficient for equilibrium to
be attained, or the kinetic rate coefficients were sufficiently large, such hys-
teretic behavior would be minimized. For the case when nonlinear kinetic
(Freundlich) sorption is considered, the respective sorption and desorption
isotherms clearly illustrate hysteresis, as shown in Figure 2.15. Reaction
times greater than 200 hours were required to achieve >90% equilibrium
when nonlinear kinetic sorption was clear. These simulations demonstrate
the concept of the influence of kinetic reaction on hysteresis regardless of the
form of the sorption retention reaction.
2.2.1 Imperial Hysteresis Coefficient
Several efforts have been made to quantify hysteresis based on adsorp-
tion and desorption parameters associated with the Freundlich equation.
Ma et al. (1993) defined hysteresis based on the difference between adsorp-
tion and desorption isotherms as a direct way to quantify the discrepancy
between sorption and desorption. They derived the following equation as a
Search WWH ::

Custom Search