Agriculture Reference
In-Depth Information
f =
1.0
Mixed Soil
Soil A = Kinetic + Irreversible
Soil B = Nonlinear Equilibrium
0.6
f =
0.75
f =
0.50
f =
0.25
0.4
f =
0
0.2
0.0
0
1
2
3
4
5
6
7
Pore Volume (V/V
o
)
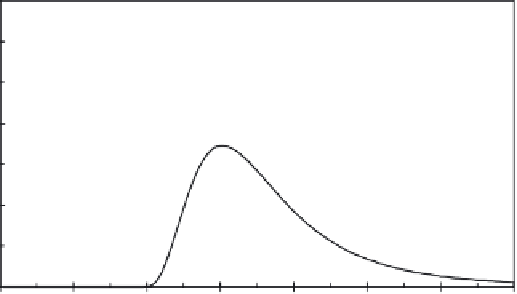
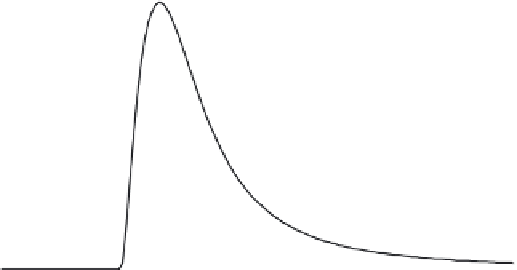
FIGURE 9.16
Miscible displacement of a solute pulse in a mixed medium made up of a mixture of two soils.
Solute retention properties of soil A include irreversible, nonlinear kinetic, and nonlinear equi-
librium reactions, whereas soil B has only nonlinear equilibrium reactions.
The second example is presented in Figure 9.16, which is for a somewhat
similar mixed medium to that shown in Figure 9.15. In this case, the added
material (soil B) is considered as a reactive material where nonequilibrium
retention conditions are dominant, that is, kinetic and irreversible processes
are ignored. This is illustrative of rapid or instantaneous sorption of a reac-
tive chemical by soil B having a nonlinear sorption isotherm. Specifically,
retention parameters selected for soil B are:
K
e
= 1 mg L
-1
,
n
= 0.5
with all
other parameters set to zero. The influence of mixing a fraction
(f)
of soil
B on the mobility of solute in this mixed medium is exhibited by the set of
BTCs shown in Figure 9.16. Lesser mobility of a solute in such a medium is
manifested by the shapes of the BTCs when compared to a mixed soil where
a nonreactive medium was added as shown in Figure 9.15. Overall, a lower
BTC peak concentration maximum and spreading is observed for the case
with nonequilibrium retention.
9.7 Second-Order Model (SOTS)
Now we will apply the second-order model for media of mixed soils.
Specifically, the dominant retention mechanisms for each soil in the mixed
system are assumed to follow that of second-order retention as previously
discussed. Briefly, the second-order formulation is based on the assump-
tion that adsorption affinities are different for the various constituents of





































Search WWH ::

Custom Search