Agriculture Reference
In-Depth Information
(NH
4
)
p
(NO
2
)
p
(N
2
O + N
2
)
g
(NO
3
)
p
K
5
K
3
K
9
K
4
K
es
K
2
K
1
(NH
4
)
e
(NH
4
)
s
(NO
2
)
s
(NO
3
)
s
K
se
KK
2
KK
7
KK
6
K
6
KK
8
(Org.N)
i
FIGURE 5.2
A schematic representation of transformation of soil nitrogen. Terms K and KK refer to rate
coefficients and e, s, p, I, and g refer to exchangeable, solution, immobilized, and gaseous
phases, respectively. (From M. Mehran and K. K. Tanji. 1974.
J. Environ. Qual.
3: 391-395. With
permission.)
and hydrologic processes controlling P transport must be clearly under-
stood. Many of the principles to be discussed for P reactions may in some
instances be applicable to other types of precipitation reactions (i.e., Al,
Fe, etc.). Mansell et al. (1977), investigating the transport of orthophos-
phate through saturated and unsaturated columns of a sandy soil, found
that a single process failed to describe P transport. Specifically, revers-
ible equilibrium adsorption-desorption relationship of the Freundlich
type inadequately described observed data. By coupling a first-order
kinetic expression with the classical transport equation and considering
nonlinear exchange of the Freundlich type, Mansell et al. (1977) substan-
tially improved the prediction of orthophosphate transport through soil
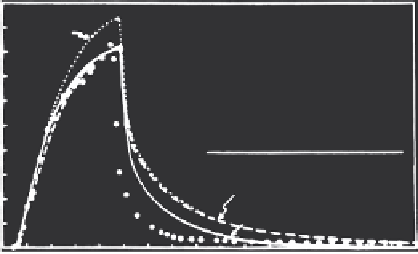
(Figure 5.3). They noted, however, that this model overpredicted the peak
1.0
No sink
0.8
-
= 42.46 cm/h
0.6
Sink term
0.4
Q = α
c
C; α
c
= 0.2 h
-1
Q = α
s
S; α
s
= 0.2 h
-1
0.2
0
0
4
8
12
16
20
24
28
32
V/V
o
FIGURE 5.3
Observed phosphate effluent condensations from the Al horizon of a sandy soil with predicted
curves determined using a one-site, nonlinear, nonequilibrium model with and without a sink
term for irreversible sorption and immobilization.





















Search WWH ::

Custom Search