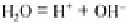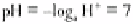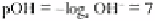Environmental Engineering Reference
In-Depth Information
in rock minerals, e.g. apatite. The third role of humus is to act as a source of most of the
micro-nutrients which plants need. Micro-nutrients are needed only in very small
quantities, but they are absolutely essential. The main micro-nutrients are iron,
manganese, copper, zinc, molybdenum and boron. Those nutrients which are also metals,
e.g. iron, manganese, zinc and copper, can be held in organic molecules in the form of
chelates
, where the metal ion is held in the form of a 'chelate ring'. The chelates will be
decomposed by micro-organisms to release the nutrient ion into the soil solution, whence
it can be absorbed by plant roots. As well as providing nutrients to plants, it must not be
forgotten that humus also supplies food for bacteria and other living organisms essential
to a productive soil. The final role of humus is its influence on the soil's physical
properties. Thus it acts to improve the soil's water-holding capacity through its effects on
soil structure (pp. 382-4). It is especially efficaceous in improving aggregation in sands
and sandy loams. Similarly, in heavy clays the humic colloids improve structure
formation and aeration. The role of humus in darkening the soil surface influences the
thermal absorption and radiation characteristics of a soil. A darker soil will heat up more
rapidly than a lighter soil, owing to its lower albedo, though it will also cool faster at
night.
pH OR SOIL REACTION
The reaction or pH of a soil greatly influences the growth of higher plants and of micro-
organisms in the soil. pH is defined as the negative index of the logarithm of the
hydrogen ion (H
+
) concentration. For pure water, the amount of dissociation into H
+
and
OH
−
ions is very small. Thus:
At 25° C the product of the ionic activities equals 10
−14
g ions (moles) litre
−1
, i.e. the
activity of each ion is 10
−7
. Hence:
and:
At neutrality, pH = pOH = 7. Thus each division below or above pH 7 represents a
tenfold decrease or increase in acidity. Natural rainwater has a pH value of about 5·5,
reflecting the pressure of carbon dioxide in the atmosphere with which the rainwater
comes into equilibrium. There is a close connection between soil pH and the degree of
base saturation
of the soil colloid complex. The higher the proportion of the cation
exchange sites which are satisfied by hydrogen (H
+
) and aluminium (Al
3+
), the lower will
be the pH. Table 18.8 illustrates the terms used to describe the increasing acidity with
lower pH and increasing alkalinity with higher pH.
Soil acidity is probably the most common and apparently simple test performed on
soil. In detail, determining the accurate level of pH is affected by a range of analytical
problems. However, field pH kits using indicators and electronic field pH meters are