Biology Reference
In-Depth Information
clonogenic capacity of CD34+ cells: the mean number of colonies was 41 ± 10
for non transduced cells and 44 ± 12 for pRMES8-transduced cells (12 animals
tested, P = 0.60 (Mann & Whitney test)). Similar results were obtained for LTC-
IC, with 19 ± 3 colonies obtained for non transduced cells and 19 ± 3 for trans-
duced cells (n = 12; P = 0.79 (Mann & Whitney test)). Transduction rates did
not differ significantly between CFC and LTC-IC (P = 0.4884 (Wilcoxon test),
n = 12), with 18% ± 7% and 19% ± 7% of colonies, respectively, eGFP-positive.
However, in both cases, the percentage of eGFP-positive cells was significantly
lower than that observed 24 hours after transduction (P < 0.0001 (Wilcoxon
test)). This apparent discrepancy between analyses carried out at 24 h and analyses
on CFC or LTC-IC may be due to the eGFP protein present in viral particles and
incorporated into the cell cytoplasm during the coculture period. The propor-
tion of cells producing eGFP shortly after transduction was reduced by 25% ±
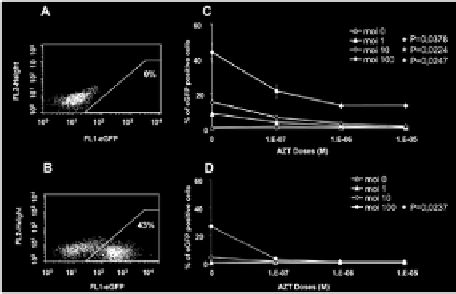
15% (Figure 2C) if 10-6 M AZT was added to cocultures of CD34+ BM cells
and lentiviral vector (MOI = 100). Untreated CFC cultures gave percentages of
eGFP-producing cells similar to those observed before differentiation (26% ±
5%) (Figure 2D). No fluorescence was detected after myeloid differentiation of
the AZT-treated CFC (n = 3), confirming that eGFP detection resulted from the
production of this protein from integrated vector.
Figure 2.
Efficiency of transduction of cynomologus macaque primitive hematopoietic cells with SIV-based
lentiviral vectors. A: Non transduced cells were used as a control for each animal. B: Transduction of bone
marrow progenitor cells with an SIV-based vector. CD34+ cells were cultured in the presence of cytokines (see
materials and methods) and exposed to vector particles at an MOI of 100 for 24 hours before FACS analysis
for eGFP production. C: CD34
+
cells were cultured overnight in a proliferation medium supplemented with
various concentrations of AZT (100 nM, 1 mM, 10 mM). Cells were then washed twice and transduced with
various multiplicities of infection (MOI) of the lentiviral vector (0, 1, 10, 100). After 24 hours of coculture
with lentiviral vector, some of the CD34
+
cells were used to evaluate the rate of transduction of undifferentiated
CD34+ cells (C); * indicate statistically significant differences (Kruskal Wallis test) between cultures with and
without AZT treatment for MOI = 1 (p = 0,0378), MOI = 10 (p = 0,0224) and MOI = 100 (p = 0,0247). Some
of the cells were cultured for 14 days, to allow the myeloid differentiation of CFC. Cells were then resuspended,
washed and fixed for three days. They were analyzed by flow cytometry, to evaluate the percentage of eGFP-
positive cells and determine the rate of transduction (D); * indicates a statistically significant difference (p =
0,0237(Kruskal Wallis test)) between cultures with and without AZT treatment for MOI = 100. The results
shown are the mean values for the three monkeys, each studied in triplicate.

Search WWH ::

Custom Search