Chemistry Reference
In-Depth Information
2000.0
0.0
-2000.0
4.00
6.00
8.00
10.00
12.00
14.00
16.00
Amount
18.00
20.00
22.00
24.00
26.00
28.00
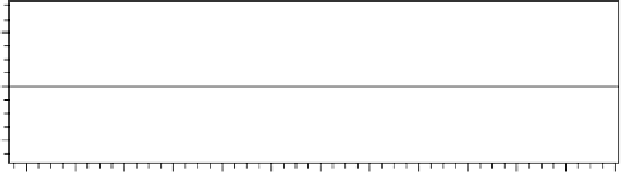
FIgure 4.7
Chromatography data system linearity plot, showing
y
-intercept, slope, and
coefficient of determination.
4.3.7 r
obuStneSS
The robustness of an analytical procedure is defined as a measure of its capacity to
obtain comparable and acceptable results when perturbed by small but deliberate
variations in procedural parameters listed in the documentation. Robustness provides
an indication of the method's suitability and reliability during normal use. During
a robustness study, method parameters are intentionally varied to see if the method
results are affected. The key word in the definition is
deliberate
. Variations should
be chosen symmetrically around a nominal value, or about the value specified in the
method, to form an interval that slightly exceeds the variations that can be expected
when the method is implemented or transferred. For example, if the buffer pH is
adjusted by titration and the use of a pH meter, the typical laboratory has an error of
≈±0.1 pH units. To test the robustness of a method to variations in a specified pH 2.5
buffer, additional buffer might be prepared and tested at pH 2.4 and pH 2.6 to ensure
that acceptable analytical results are obtained. For instrument settings, manufactur-
ers' specifications can be used to determine variability. The range evaluated during
the robustness study should not be selected to be so wide that the robustness test will
purposely fail, but rather to represent the type of variability routinely encountered in
the laboratory. Challenging the method to the point of failure is not necessary. One
practical advantage of robustness tests is that once robustness is demonstrated over
a given range of a parameter, the value of that parameter can be adjusted within that
range to meet system suitability without a requirement to revalidate the method.
Robustness should be tested late in the development of a method, and if not, is
typically one of the first parameters investigated during method validation. However,
throughout the method development process, attention should be paid to the identifi-
cation of which chromatographic parameters are most sensitive to small changes, so
that when robustness tests are undertaken, the appropriate variables can be tested.
Robustness studies also are used to establish system suitability parameters to make
sure the validity of the entire system (including both the instrument and the method)
is maintained throughout method implementation and use. In addition, if the results
of a method or other measurements are susceptible to variations in method param-
eters, these parameters should be adequately controlled and a precautionary state-
ment included in the method documentation.