Chemistry Reference
In-Depth Information
9.4.1.4 verification process examples
Assessing specificity is often critical to verifying that a compendial procedure is
suitable for use in assaying drug substances and drug products. Specificity for a
chromatographic method may be verified by conformance with system suitability
resolution requirements if they are specified in the method. However, drug sub-
stances from different suppliers may have different impurity profiles that are not
addressed by the compendial test procedure. Similarly, the excipients in a drug
product can vary widely among manufacturers and may have the potential to
directly interfere with the procedure or cause the formation of impurities that are
not addressed by the compendial procedure. In addition, drug products containing
different excipients, antioxidants, buffers, or container extractives may potentially
interfere with the compendial procedure. In these cases, a more thorough assess-
ment of specificity may be required to demonstrate suitability of the method for the
particular drug substance or product, for example, to include photodiode array and
mass spectral analysis.
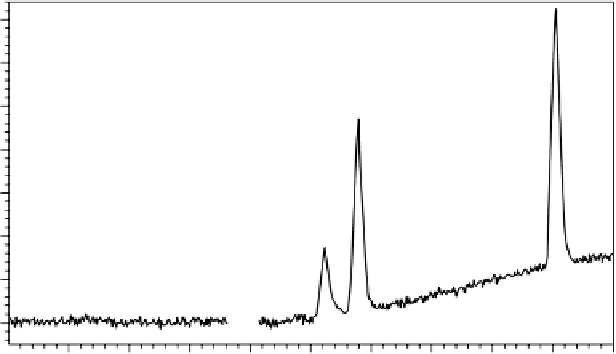
Figure 9.1 shows a separation used to verify a stability indicating compendial
procedure for the analysis of a drug product and its major degradants. In addition to
specificity, precision and the quantitation limit were also evaluated. Specificity was
evaluated using photodiode array peak purity; Table 9.1 summarizes the precision
results, and Table 9.2 the results from the determination quantitation limit. Figure 9.1
illustrates the actual separation at the quantitation limit used to verify the calculated
quantitation limit.
0.007
4
4.531
0.006
3
0.005
2.893
0.004
0.003
2
0.002
2.621
1
1.912
0.001
0.000
0.50
1.00
1.50
2.00
2.50
Minutes
3.00
3.50
4.00
4.50
5.00
FIgure 9.1
Verification of compendial procedure quantitation limits. Separation was
performed on an Alliance 2695 Separations Module (Waters, Milford, Massachusetts) using
a 4.6 by 100 mm 3.5-µ Xterra RPC18 column at 34°C. Mobile phase A was 10 mm pH 9.0
ammonium carbonate, B was methanol run at a 15%-90% B linear gradient over 5 min at 1.0
mL/min. A 20-µL injection and UV detection at 280 nm were also used. Peaks are in order:
(1) NTAP (highlighted in red), (2) ACBS, (3) HCT, and (4) TMT.