Chemistry Reference
In-Depth Information
IND
NDA
Submissions
Drug Discovery
Preclinical
Clinical Trials
FDA
Review
MFG
MKTNG
Phase
One
Phase
Two
Phase
ree
5,000-10,000
Compounds
250
Compounds
Compounds
Approved
Five Compounds
One
Number of Volunteers
100-
500
1000-
5000
20-100
Six Mos.
to Two
Years
ree to Six Years
Six to Seven Years
Non-Regulated
Regulated GLP/GMP
Target ID
Lead ID
Screening
Optimization
Process R&D
Formulation
Metabolism
Toxicology
Scale-up
Pharmacokinetics
Delivery
Safety
Production
QA/QC
Legal
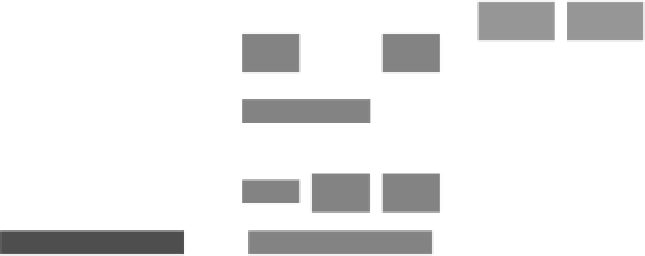
FIgure 1.1
An overview of the stages in the drug development process.
regulated GMP fashion. It is at this point, following the Preclinical Phase studies,
that the Investigational New Drug (IND) application is made to the FDA.
In the clinical phase, containing Phase I-III safety and efficacy studies, there
will be human pharmacokinetics studies, which again may need additional method
development and validation work to be performed due to the different matrices that
might be involved. At this point in time, while the drug moves closer to market,
a New Drug Application (NDA) filing is prepared that includes a complete AMV
package according to the type of method and its intended use. Complete validation
at this point in the process might also include interlaboratory collaborative studies
(also known as round-robin studies), involving a number of labs, analysts, instrumen-
tation, and samples to prepare for the transfer of the method, depending on where or
how it is implemented.
In the end, the amount or extent of method validation can be correlated with
Figure 1.1; that is, the amount of validation increases the further a drug moves along
in the development process [2,3]. One of the major goals in method validation is to
balance the amount of validation performed to meet United States Pharmacopeia
(USP) guidelines and FDA recommendations. As the drug survives the stages indi-
cated in Figure 1.1 and moves toward marketing approval, there is no need to per-
form a comprehensive or complete validation for a new method on a drug that is
early in the discovery or preclinical stages of its life cycle. In early development,
only minimal validation work is performed; and if the drug survives these early
stages, the amount of validation performed will increase as the drug moves closer to
market. Therefore, AMV is an evolving process, largely dependent on where a given
drug is in its stages of development.