Chemistry Reference
In-Depth Information
55.6
0.0
0
1000
2000
Concentration (ng/µL)
3000
4000
5000
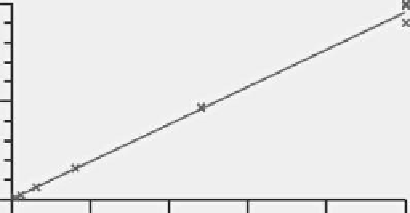
FIgure 7.11
Example calibration plot obtained for the LC-MS/MS analysis of standard
at 10, 30, 100, 300, 800, 2400, and 5000 ng/L. Column: 100 mm 2-mm C18; mobile phase:
55% acetonitrile, 45% water, 0.1% formic acid; flow rate: 0.3 mL/min; injection volume:
20 µL. MS detection: Quatro Ultima (Waters Corp.) MS/MS with a positive-ion electrospray
Z source; cone voltage: 100 V; collision energy: 18 eV; collision gas pressure: 2.5 mbar
argon.
Because a significant amount of sample manipulation takes place in the typical sam-
ple preparation procedure, internal standards are preferred for most bioanalytical
methods. At least four out of six nonzero standards (67%) should fall within ±15%
of the nominal concentration (±20% at the LLOQ). The calibration curve should be
generated for every analyte in the sample, and prepared in the same matrix as the
samples by addition of known concentrations of the analyte to blank matrix. The
FDA guidelines suggest that a calibration curve should be constructed from six to
eight nonzero samples that cover the expected range, including the LLOQ. In addi-
tion, noninterference is shown by the analysis of a blank sample (nonspiked matrix
sample processed without internal standard) and a zero sample (nonspiked matrix
processed with internal standard). Two conditions must be met to determine the
LLOQ: (1) analyte response at the LLOQ should be >5-times the blank response,
and (2) the analyte peak should be identifiable, discreet, and reproducible with an
imprecision of ≤20% and an accuracy of at least 80%-120%. Figure 7.11 shows an
example calibration curve for an LC-MS/MS experiment.
7.5.2.4 bioanalytical sample stability
Stability
tests determine that the analyte (and internal standard) does not break down
under typical laboratory conditions, or if degradation occurs, it is known and can be
avoided by appropriate sample handling. Many different factors can affect bioana-
lytical sample stability; these include the chemical properties of the drug, the stor-
age conditions, and the matrix. Studies must be designed to evaluate the stability of
the analyte during sample collection and handling, under long-term (at the intended
storage temperature) and short-term (benchtop, controlled room temperature) stor-
age conditions, and through any freeze-thaw cycles. The conditions used for any
sample-stability studies should reflect the actual conditions the sample may experi-
ence during collection, storage, and routine analysis, including working and stock