Chemistry Reference
In-Depth Information
Bioanalytical methods must be validated to demonstrate that they are reliable and
reproducible for their intended use (as for any other analytical method).
Analytical methods for finished product, raw materials, or active pharmaceuti-
cal ingredients (APIs) each have their own development and validation challenges.
Bioanalytical methods are further complicated by the nature of the sample matrices,
the trace concentrations of drug and metabolites encountered, and (potentially) the
complexity of the required instrumentation.
Preclinical and clinical pharmacology studies rely on the sensitivity and selectiv-
ity of bioanalytical methods. Industry and regulatory conferences have been held
over the past several years to discuss bioanalytical method validation [33-35], and
after two early conferences, in May of 2001 the FDA issued a guidance document for
validating bioanalytical methods [36]. Bioanalytical method regulations are listed as
“guideline”, the general interpretation of these guideline documents is that if meth-
ods are developed that adhere to their recommendations, there will be less likeli-
hood of a negative regulatory action. In other words, if the recommendations of the
guidelines are
not
followed, you should be sure to develop a logical and scientifically
supported statement to show that alternative performance criteria are justified.
Regulated bioanalysis usually involves an HPLC system coupled to a triple-
quadrupole mass spectrometer (LC-MS/MS). The sensitivity and selectivity of the
LC-MS/MS allows for the quantitation of analytes with acceptable precision and
accuracy at concentrations lower than most other HPLC detectors. Short, small-
particle columns (e.g., 30-50 × 2.1 mm i.d. packed with ≤3-μm particles) are typi-
cally used for the fast separations needed for the large number of samples generated
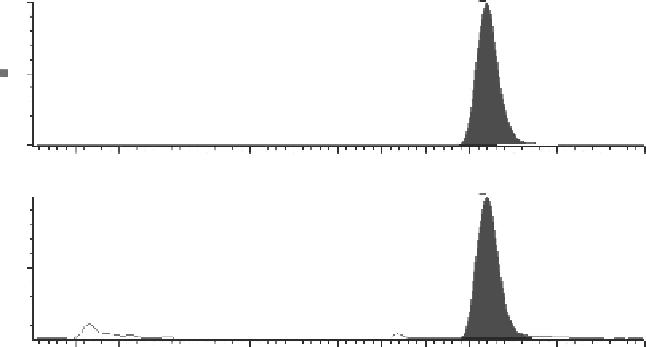
by clinical studies. An example is presented in Figure 7.10. Isocratic or gradient tech-
niques may be used with run times commonly less than 5 min. Sample preparation
QC 35
GLPQUAN00195 Sm (SG, 2×2)
MRM of 2 Channels ES+
512.2 > 424.2
4.88e5
Area
2.59
71223
100
%
0
GLPQUAN00195 Sm (SG, 2×2)
MRM of 2 Channels ES+
506.2 > 224.9
1.71e5
Area
2.59
25475
100
%
0
0.50
1.00
1.50
2.00
2.50
3.00
3.50
Time
FIgure 7.10
Example LC-MS/MS chromatogram used to generate the plot in Figure 7.11.