Chemistry Reference
In-Depth Information
100
90
80
70
120
100
*Q Factor A
80
60
40
30
20
80
*Q Factor A
60
40
6 0 6 0 6
Transfer Time (min)
(a)
30
36
40
46
50
200
300
400
500
Transfer Time (min)
(b)
600
700
800
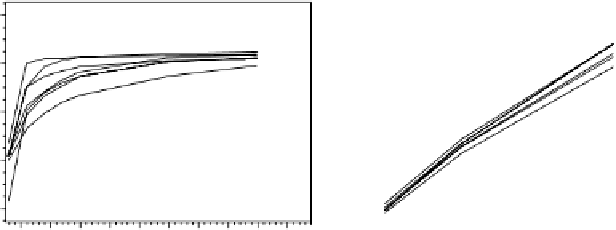
FIgure 7.9
Example dissolution rate release curves. (a) Phenylpropanolamine HCl
immediate-release tablets. Six vessel dissolution test conditions: Apparatus 2 (paddle) at
50 rpm, Q factor >75% released at 45 min. Assay was performed by reversed-phase HPLC.
(b) Phenylpropanolamine HCl extended-release tablets. Six vessel dissolution test condi-
tions: Apparatus 1 (basket) at 100 rpm, Q factor >70% released at 720 min. Assay was
performed by reversed-phase HPLC.
example, an extended-release formulation, and are typically in the range of 75% to
80% dissolved. A Q value in excess of 80% is not generally used, because allow-
ance must be made for assay and content uniformity ranges. Figure 7.9 illustrates
some example rate release dissolution profiles for both an immediate-release and an
extended-release formulation of the same drug substance as determined by HPLC
analyses.
Finally, the dissolution test procedure should be discriminating enough to be
capable of distinguishing significant changes in a composition or manufacturing
process that might be expected to affect in vivo performance. In general, a properly
designed dissolution test should result in reproducible data. Too much result vari-
ability can make it difficult to identify trends, true batch differences, or effects of
formulation changes. If too much variability is observed, the usual remedies include
changing the apparatus type, speed of agitation, or deaeration; consideration and
examination of sinker type; and changing the composition of the medium. During
routine testing of the product, variability outside the expected range should be inves-
tigated from analytical, formulation, and processing perspectives.
7.4.2.4 Assaying the results
There are two common ways of analyzing dissolution test samples: spectrophoto-
metric (UV) determinations and HPLC. UV determinations are the most common
method of analysis because they are faster, simpler, and require less solvent than
HPLC. Typically, the drug substance UV spectrum is observed to choose the opti-
mum wavelength for analysis. Cells with path lengths ranging from 0.02 to 1 cm are
typically used; the smaller path length cells are used to avoid diluting the sample
once acceptable linearity and standard error are demonstrated.
HPLC methods, however, have distinct advantages, particularly when there is
significant interference from excipients or between multiple active ingredients in the