Chemistry Reference
In-Depth Information
knowledge as it relates to the API structure, in order to achieve these degradations.
After API partial degradation, the samples are quenched, if necessary, to stop fur-
ther reaction, and are analyzed to ensure that all impurity and degradants peaks are
resolved and all degradants are resolved from each other and the API (utilizing peak
purity analyses approaches, as described earlier).
Only the combination of both PDA and MS on a single instrument and software
platform provides the type of valuable orthogonal information required when evalu-
ating specificity and developing SIMs.
7.3.1.5 new technology for sIm development
Resolving power, specificity, and speed are key chromatographic method attributes
to keep in mind during SIM development. Recently, new chromatographic technol-
ogy has been introduced that capitalizes on small, 1.7-µm particle column packings
that can dramatically impact the analysis (method development and validation) of
degradation products by providing much-improved resolution and sensitivity [21-
24]. By using smaller particles, speed and peak capacity (number of peaks resolved
per unit time in the gradient mode) can be extended to new limits, termed Ultra High
Performance Liquid Chromatography, or UHPLC. Using UHPLC, it is possible to
take full advantage of chromatographic principles to run separations using shorter
columns or higher flow rates for increased speed with superior resolution and sensi-
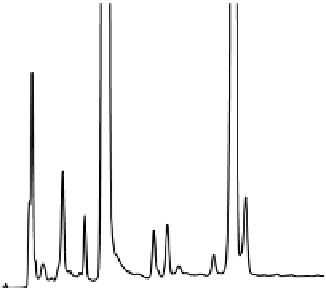
tivity, important attributes for SIMs. Figure 7.7 is an example of a SIM developed for
the analysis of a drug-product-related degradation study.
0.070
1
2
0.060
0.050
0.040
0.030
0.020
0.010
0.000
0.50
1.00
1.50
2.00
2.50
3.00
3.50
4.00
Minutes
FIgure 7.7
UHPLC separation of a hydrocodone/acetaminophen formulation subjected
to forced degradation at accelerated temperature. A 1.7-μm 2.1 by 50 mm ACQUITY UPLC
BEH C
18
Column (Waters, Milford, Massachusetts) at 30°C was used, and a 3%-20%B lin-
ear gradient over 0.8 min, at a flow rate of 0.8 mL/min. Mobile phase A was pH 2 sodium
phosphate; B was acetonitrile. UV detection at 233 nm. Injection volume 5.0 µL. Peak 1 is
hydrocodone; peak 2 is acetaminophen. The remaining peaks are unidentified degradation
products. (Courtesy of Waters Corporation.)