Chemistry Reference
In-Depth Information
0.010
0.010
1X ICH
Acid
0.008
0.008
0.006
0.006
0.004
0.004
0.002
0.002
0.000
0.000
-0.002
-0.002
1.00
2.00
3.00
4.00
5.00
6.00
7.00
8.00
1.00
2.00
3.00
4.00
5.00
6.00
7.00
8.00
Minutes
Minutes
0.010
0.010
3X ICH
Base
0.008
0.008
0.006
0.006
0.004
0.004
0.002
0.002
0.000
0.000
-0.002
-0.002
1.00
2.00
3.00
4.00
Minutes
5.00
6.00
7.00
8.00
1.00
2.00
3.00
4.00
Minutes
5.00
6.00
7.00
8.00
0.010
0.010
Peroxide
Heat
0.008
0.008
0.006
0.006
0.004
0.004
0.002
0.002
0.000
0.000
-0.002
-0.002
1.00
2.00
3.00
4.00
Minutes
5.00
6.00
7.00
8.00
1.00
2.00
3.00
4.00
Minutes
5.00
6.00
7.00
8.00
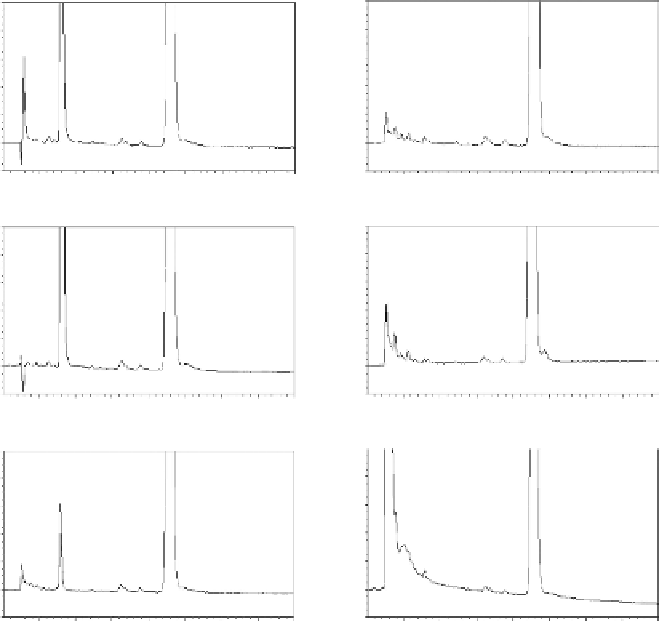
FIgure 7.1
Example chromatographic comparison of forced degradation experiments.
Chromatographic conditions used an isocratic mobile phase of 45/55 ammonium acetate
pH 4.5/acetonitrile at 0.6 mL/minute. Column was a 3.0 by 75 mm 2.7 µm C
18
Halo (MAC-
MOD Analytic, Chadds Ford, Pennsylvania). A 5-µL injection and UV detection at 238
nm were used. Test compound is simvastatin, subjected to the kind of forced degradation
conditions outlined in Table 7.4.
products might be missed. Experience and data obtained from studies performed
previously on related compounds should also be used when developing new proto-
cols. Figure 7.1 illustrates some comparison chromatograms of a forced degradation
study using conditions similar to those outlined in Table 7.4.
7.3.1.2 developing the lc method
Once the sample is generated through the use of a properly designed and executed
forced degradation, it can be used to develop the HPLC method. Nowadays, HPLC
method development is often performed on gradient systems capable of automated
column and solvent switching, and temperature control. Systems and software that
automate the process, some with decision making built in, have also been reported
[14,15]. Scouting experiments are often run, and then conditions chosen for further