Chemistry Reference
In-Depth Information
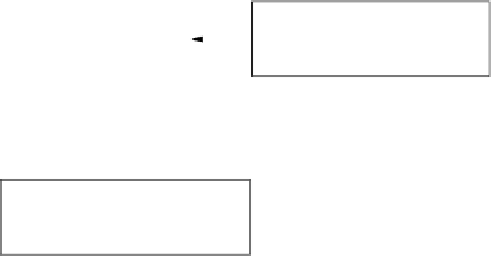
Determine impurity level in
relevant batches
1
Is
impurity also
a degradation
product?
Estimate maximum increase in
impurity at retest date using data
from relevant accelerated and
long-term stability studies
Determine mean + upper
confidence limit for the impurity
(Let this = A)
Ye s
No
Is
A or B
greater than the
qualified
level?
Determine maximum likely level as:
A + increase in degradation product
at appropriate storage conditions.
(Let this = B)
No
Acceptance criterion = A or B
(as appropriate)
Ye s
Acceptance criterion = qualified
level or establish new qualified level
2
FIgure 6.1
Establishing acceptance criterion for a specified impurity in a new drug sub-
stance.
1
Relevant batches are those from development, pilot, and scale-up studies.
2
Refer to
ICH guidelines on Impurities in New Drug Substances [6].
that falls outside the specification or acceptance criteria. While steps can be taken to
decrease the frequency of OOS (out of specification) results, it is rare that they can
be completely prevented. FDA regulations require that an investigation be conducted
whenever an OOS test result is obtained. Therefore, it is essential in a regulated
laboratory to have a standard operating procedure (SOP) in place that describes the
actions to take to determine the cause of the OOS result, and the corrective action
that must be undertaken. A thorough SOP will ensure that correct decisions are
made regarding the acceptance or rejection of a batch. And batch rejection does not
negate the need to perform an investigation. Thorough and systematic investigation
of an OOS result not only leads to scientifically sound decisions, but also is man-
dated by law in the Code of Federal Regulations (CFR) and by the court's decision in
the now-infamous 1993 case of U.S. FDA versus Barr Labs. Indeed, FDA guidance
is available on the topic of OOS investigations; and while this chapter will discuss
the FDA guidance in some detail, the reader is encouraged to consult the references
for additional details [14,15]. FDA guidance documents are always a good source
of information because they are prepared for review staff and establish policies
intended to achieve consistency in the FDA's policy and regulatory approach, and