Chemistry Reference
In-Depth Information
HPLC
0.40
0.30
0.20
0.10
0.00
0.00
5.00
10.00
15.00
20.00
Time (min)
(a)
25.00
30.00
35.00
0.40
UHPLC
0.30
0.20
0.10
0.00
0.00
0.50
1.00
1.50
2.00
Time (min)
(b)
2.50
3.00
3.50
4.00
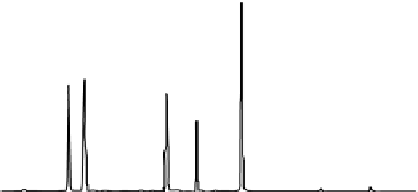
FIgure 5.6
Separation properly scaled from a (a) 5.0-µm to a (b) 1.7-µm column. (a)
Original HPLC separation of caffeic acid derivatives from
Echinacea purpurea
, a natural
product. Column: 4.6 by 150 mm, 5.0-µm XTERRA
®
MS C
18
Column (Waters Corporation)
at 40°C. A 8-50%B linear gradient over 32 min, followed by a 3-min step to 90%B, and a
6 min reequilibration to starting conditions, at a flow rate of 1.0 mL/min, was used. Mobile
phase A was 0.1% CF
3
COOH in H
2
O, Mobile Phase B: 0.08% CF
3
COOH in acetonitrile, UV
detection at 330 nm. Peaks are in order: Caftaric acid, chlorogenic acid, cynarin, echina-
coside, cichoric acid, 0.1 mg/mL each in 50:50 H
2
O: MeOH with 0.05% CF
3
COOH, 10 µL
injection. (b) Resulting 1.7-µm particle separation of caffeic acid derivatives from
Echinacea
purpurea
, a natural product, after scaling the HPLC separation. Column: 2.1 by 50 mm
1.7-µm ACQUITY™ BEH C
18
Column at 40°C. A 8-50%B linear gradient over 4.45 min,
followed by a 0.41 min step to 90%B, and a 1.14-min reequilibration to starting conditions, at
a flow rate of 0.5 mL/min, was used. Sample and mobile phase conditions were identical to
Figure 5.1a. A 1.0-µL injection was used.
5.10 conclusIon
A properly designed, executed, and evaluated robustness study is a critical
component of any method validation process. In a development laboratory, a robust-
ness study can provide valuable information about the quality and reliability of
the method, and is an indication of how good a job was done in developing and