Agriculture Reference
In-Depth Information
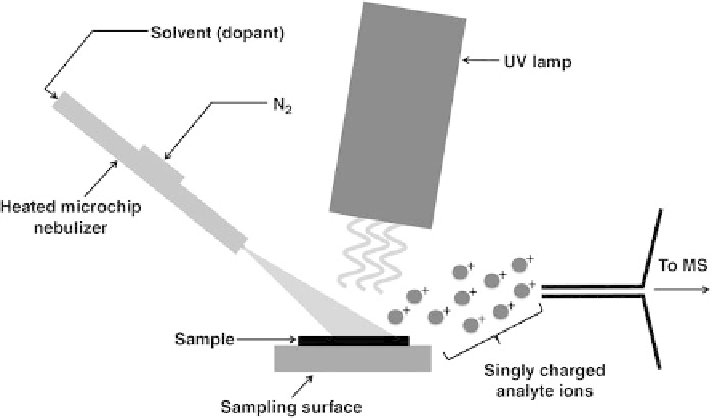
Figure 2.12.
A scheme of DAPPI ion source and ionization process.
xyz
-stage. The thermally desorbed vapors, containing both dopant and sample compo-
nents, are subsequently irradiated by UV light and photoionization of analytes occurs
(Figure 2.12). While the UV-absorbing analytes can be ionized directly, compounds
lacking the chromophore group cannot undergo direct ionization and are ionized
through molecule
ion interactions with dopant and atmospheric water ions [113]. The
DAPPI technique was shown to be capable of ionizing both polar and nonpolar
compounds. The nature of the particular analyte and the dopant solvent dictates the type
and intensity of ions formed in DAPPI. In positive ion mode, the spraying solvents that
yield radical cations upon photoionization (e.g., toluene) can be used for ionization of
low-polarity, low proton af
-
nity analytes (M
+
ions), while the solvents generating the
proton-donating reactive species (e.g., acetone, methanol, or hexane) can protonate
high proton af
H]
+
ions. In negative ion DAPPI,
solvents with ionization energies below the energy of UV lamp photons provide
the best ionization ef
nity compounds to form [M
+
H]
and M
ion. Other
important factors affecting the ionization yield are related to source geometry and
thermal conductivity of the sampling surface. Materials with low thermal conductivity,
such as poly(methyl methacrylate) (PMMA) or polytetra
ciencies in the formation of [M
uoroethylene (PFTE), can be
locally heated to higher temperatures, which lead to improved ef
ciency of the
thermodesorption process [114].
2.3.3.2 Optimization of DESI-MS and DART-MS-Based Methods
Although widely perceived as a simple and straightforward approach, successful
application of ambient MS to an analytical problem typically requires careful
optimization of many parameters. This need is even more pronounced in cases in