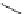Agriculture Reference
In-Depth Information
OH
OH
H
2
N
NH
2
NH
2
OH
O
O
NH
+
O
H
2
O
O
H
O
H
OH
H
OH
H
O
H
OH
O
OH
OH
Asparagine
CO
2
Glucose
OH
O
N
NH
NH
NH
2
O
N
NH
2
H
2
C
O
O
OH
H
Acrylamide
OH
Figure 4.1.
Formation of acrylamide in food processing.
acid asparagine and reducing sugars such as glucose and fructose at elevated
temperatures and low-moisture conditions
a process known as the Maillard reaction
(Figure 4.1) [27,28]. Acrylamide is therefore formed in the production of chips and
crisps and is among a series of contaminants, including furan, which can be produced
during food manufacturing.
There are two main techniques used for the analysis of acrylamide by laboratories
all over the world: LC
—
MS, there are two approaches:
the use of complicated multiple solvent extractions [29] producing detection limits
that are above 10
-
MS/MS and GC
-
MS. In GC
-
g/kg and can be affected by matrix, and the use of bromination [30]
that offers adequate sensitivity with multiple ion con
μ
rmation. However, in the use of
GC, there is a risk of a false positive result because of acrylamide formation in the hot
GC injector if the reducing sugars and asparagine are present in the food extract.
Determination of acrylamide using LC
MS/MS, therefore, offers an alternative
approach where potential injector formation of acrylamide does not occur while
providing lower detection limits and avoiding a time-consuming derivatization step
that is sometimes needed for GC
-
-
MS.
MS/MS methods often use a simple solvent extraction followed by dilution to
minimize possible matrix effects [31]. The sample is then analyzed using a fast LC
separation with accurate and reproducible MS/MS detection allowing quantitation
down to low
LC
-
MS/MS methods
tend to use electrospray ionization (ESI) in positive polarity mode with multiple
reaction monitoring (MRM) and typical Q1/Q3 mass transitions of 72/55 and 72/44.
A reversed-phase column using a polar endcapped stationary phase [32] or a
Hypercarb phase [31] designed to retain small polar compounds is used in order
to retain the acrylamide. To increase sensitivity, volatile acids such as formic acid [31]
or acetic acid [32] are added to the mobile phase and acrylamide is eluted using a
gradient from aqueous to higher levels of methanol. When the more retentive
Hypercarb phase [31] is used, gradient elution with methanol is also used. When
g/kg (ppb) levels in food, as shown in Figure 4.2. LC
-
μ