Environmental Engineering Reference
In-Depth Information
potentials sufficiently separated from each other to provide an electromotive
force to drive the oxidation-reduction reactions needed to charge and dis-
charge the cell.
9
Energy is stored chemically in different ionic forms of vana-
dium in a dilute sulfuric acid electrolyte. The electrolyte is pumped from
separate storage tanks into flow cells across a proton exchange membrane
where one form of the electrolyte is electrochemically oxidized and the other
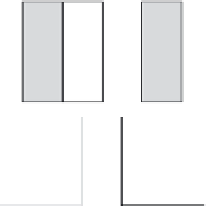
is electrochemically reduced (Figure 6.5).
The two electrolytes do not mix together; they are separated in the cells by
an extremely thin membrane that allows only selected ions to flow through.
The redox reactions take place in the cells on inert carbon felt polymer com-
posite electrodes and create a current that becomes available to do work
through an external circuit, after which charging the battery can reverse the
reaction.
Before the VRB appeared, the main disadvantage to flow batteries was that
the two liquid electrolytes were made of different substances and separated
by a thin membrane that was eventually permeated, after which the two
substances would mix and render the battery useless. The main advantage of
the VRB system is that vanadium is present in both the positive and negative
electrolytes, but in different oxidation states. Vanadium has four oxidation
states: V
+2
, V
+3
, V
+4
, and V
+5
. The VRB thereby exploits the ability of vanadium
to exist in solution in four different oxidation states—an ability shared only
with uranium and other heavy radioactive elements.
Ion-selective
membrane
Electrode
-
+
Electrolyte
Electrolyte
Flow
Cell
Power source/load
FIGURE 6.5
Flow battery that stores energy in liquid electrolytes. (Courtesy of VRB Power Systems Inc.)