Biomedical Engineering Reference
In-Depth Information
The mechanical load in paralytic disuse was assumed to decrease exponentially with time:
Ft
()
0
=⋅
Fe
ta
−⋅
(25.12)
with
t
= time (year), and
a
as a decreasing coefficient, which was chosen such that the simulation
results could be in accordance with experimental data ranges (Eser et al., 2004).
F
0
was the equi-
librium load.
Using a time-dependent approach (Equations 25.3, 25.5, 25.6, 25.10, 25.11, and 25.12), computer
simulations of cortical endosteal remodeling processes in the femur, as well as in the tibia, were
performed from
t
= 0 days to
t
= 50 years to cover the course of the clinical investigation.
25.3
reSultS From the model analySIS
25.3.1 r
eSultS
for
t
raBecular
B
one
l
oSS
a
SSociated
WitH
m
ecHanical
d
iSuSe
and
e
StroGen
d
eficiency
In the animal experiment by Lecoq et al. (2006), the bone mineral density of the distal femoral
metaphysis was measured by dual x-ray absorptiometry in all animals on days 0, 7, 14, and 30.
The differences between the experimental groups and the control groups were compared with the
simulation results.
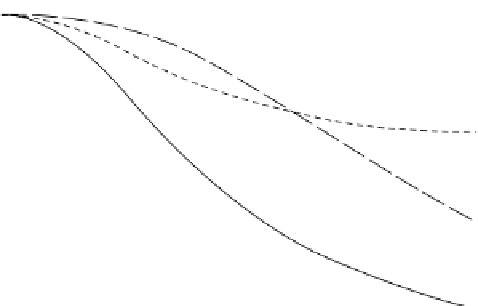
Figure 25.2 shows the predicted bone losses due to mechanical unloading, estrogen deficiency,
or both over the 30-day experimental period. The data points were calculated from a comparison of
the experimental groups and control groups in Lecoq et al. (2006). The simulated bone loss patterns
due to mechanical disuse, estrogen deficiency, or both all corresponded well with the experimental
observations.
Mechanical unloading
Estrogen deficiency
*
Both factors
5
0
-5
-10
-15
-20
-25
-30
0
5
10
15
Time (d)
20
25
30
FIgure 25.2
Simulation results for the percent change in BMD due to mechanical disuse, estrogen defi-
ciency, or both. (Data from Lecoq et al.,
Joint Bone Spine
, 198-95, 2006.)


Search WWH ::

Custom Search