Biomedical Engineering Reference
In-Depth Information
F
Periosteal surface
Endosteal surface
R
r
h
0
h
l
F
FIgure 25.1
A simplified model for cortical bone remodeling simulation. The remodeling analysis was
performed on a representative rectangular slice of the cross section of the cortical bone volume, as shown
schematically here. Bone material was assigned as linearly elastic and isotropic. Axial compressive loads
F
were placed on the cortical bone volume. For simplification, the cortical bone volume was assumed to be
cylindrical.
r:
Initial endosteal radius;
R:
periosteal radius;
l
: length of the representative rectangular slice;
h
:
cortical thickness; and
h
0
: initial cortical thickness with
h
0
=
R
-
r
.
25.2.2
m
odel
V
alidation
25.2.2.1 model validation for the Computational
Simulation of trabecular bone remodeling
The computational simulation algorithm of trabecular bone remodeling (Equations 25.1-25.8) was
validated through an animal experiment performed by Lecoq et al. (2006). Thirty-six 12-week old
female Wistar rats were randomized into groups with (1) bilateral surgical ovariectomy without
tail suspension for comparison with the estrogen deficiency simulation model; (2) bilateral surgical
ovariectomy with tail suspension for comparison with the dual-factor simulation model; (3) sham
surgery with tail suspension for comparison with the mechanical unloading simulation model; or (4)
sham surgery without tail suspension as the control group.
Time-dependent computer simulations using Equations 25.1-25.8 of the bone remodeling pro-
cess were performed on a representative cross section of 6 mm
2
trabecular bone in the distal femoral
metaphysis of the rats from
t
= 0 day to
t
= 30 day to model the experimental time.
The nominal values of the constants in the model are listed in Table 25.2. Gong et al. (2006) sug-
gested that the BMU activation threshold increased due to estrogen deficiency and the mechanical






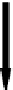








































Search WWH ::

Custom Search