Geology Reference
In-Depth Information
3 kbar
Phl
En
Fo
San
HO
2
CH
4
E
n
dry
CO
2
F
Phl
Ks
Fo
Fig. 12.15 Part of the join kalsilite-forsterite-quartz showing relative positions of the enstatite-
forsterite phase boundary in the presence of various volatile species. The boundary for CH
4
is
estimated from its position in the nepheline-forsterite-quartz system, taken from Gupta et al.
(1987). The position with HF is uncertain, but can be expected to be close to the CH
4
position. The
data for the water-saturated kalsilite-forsterite-quartz join at 0.3 GPa are taken from Luth (1967)
(after Foley et al. 1986). Abbreviations as in Fig.
12.14
enstatite + forsterite phase boundary is in a similar position to that in the CO
2
-
saturated join (Fig.
12.12
) of Gupta and Green, 1988. The position of this phase
boundary is frequently taken as the degree of polymerization of the melt (Eggler
1974; Kushiro 1975). Expansion of the enstatite phase volume at the expense of
forsterite with the addition of
fluorine causes
polymerization of the structure. The assemblage phlogopite + enstatite + forste-
rite + liquid in the simple join is similar to that of a phlogopite harzburgite. The
position of this point may indicate that partial melting of a phlogopite harzburgite in
H
2
O-free conditions but
fluorine therefore, suggests that
fluorine should produce a silica-
undersaturated magma. Such a melt should lie to the silica-poor side of the for-
sterite-sanidine join at 1,480
in the presence of
°
C. In contrast, in the water-bearing system (Gupta and
Green 1988), the
first melt would lie in the more silica-saturated part of the system,
within the triangle, de
C.
Foley et al. (1986) conducted several experiments with composition 1
(Fig.
12.14
) with 10 % F
2
O
−
1
. They observed that the near-liquidus runs contained
a very minor amount of immiscible liquid phase rich in Mg and F. The immisci-
bility may extend above the liquidus, but this could not be ascertained because of
the abundance of quench crystals in these near liquidus runs. They further observed
that with 10 % F
2
O
−
1
, composition 1 lies just inside the primary phase volume of
phlogopite, indicating expansion of the phlogopite phase volume with increasing
ned by forsterite, enstatite and sanidine at 1160
°
fluorine, They think that because of the apparent immiscibility, which occurs at
high
fluorine contents, it is unlikely that phlogopite will melt congruently at any
fluorine content.
















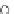












Search WWH ::

Custom Search