Geology Reference
In-Depth Information
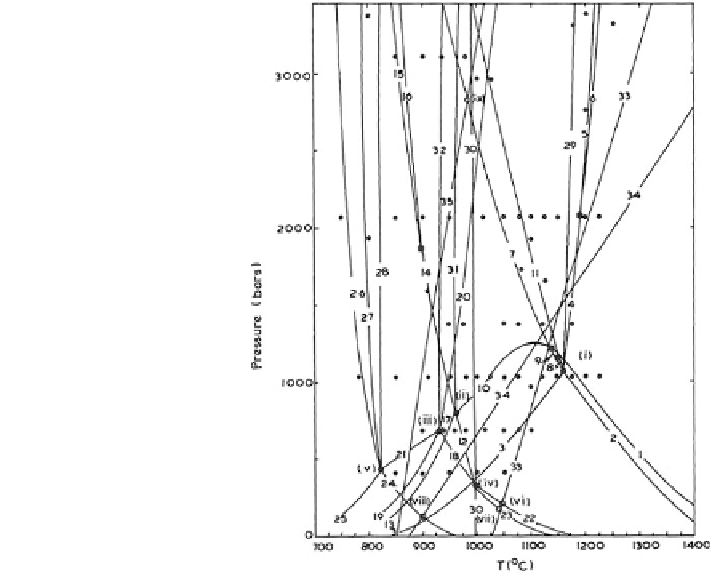
Fig. 12.1 P-T Projection of
inferred invariant and
univariant equilibria in the
system
KAlSiO
4
-
Mg
2
SiO
4
-
H
2
O.
The lower case Roman
numerals and Arabic
numerals refer to invariant
and univariant equilibria,
given in Table
12.1
. The
positions of
is from Wones
(after Luth 1967)
ν
with a water-saturated silicate melt. The P-T condition of various inferred phase
diagrams (Fig.
12.2
a
i) can be obtained from Fig.
12.2
j.
-
i shows that at pressures at or below 0.2 GPa there are liquids of
two different compositions. One is silica-undersaturated and coexists with ortho-
rhombic kalsilite, leucite, forsterite (or phlogopite) and vapour, and the other is
silica-saturated in equilibrium with K-feldspar, quartz, enstatite (or phlogopite), and
vapour. According to Luth (1967),
Figure
12.2
a
-
ed equivalents of
phonolite, rhyolite, and trachyte, respectively. Comparison of Fig.
12.2
a with
Fig.
12.2
b
these liquids are simpli
j shows that at or above 0.1 GPa phlogopite appears as a phase in this
system, whereas near 500 bar it completely disappears by reaction with a liquid.
Bowen (1928) considered that a reaction between mica and a ma
-
c liquid have
played an important role in the genesis of potassium-rich undersaturated liquid.
Figure
12.2
a
i show that forsterite is a liquidus phase in a wide range of
compositions. With further variation of bulk compositions, leucite, K-feldspar,
enstatite and/or phlogopite may join olivine in the course of equilibrium crystalli-
sation. At a pressure of less than 300 bar (near the surface) the resulting crystal
assemblage should consist of forsterite, leucite, and enstatite with siliceous inter-
stitial liquid. At slightly higher water pressures, forsterite would cease to exist
(depending on bulk compositions), and phlogopite and leucite, phlogopite and
enstatite, or phlogopite and K-feldspar should be stable. In the case of natural
-
Search WWH ::

Custom Search