Geology Reference
In-Depth Information
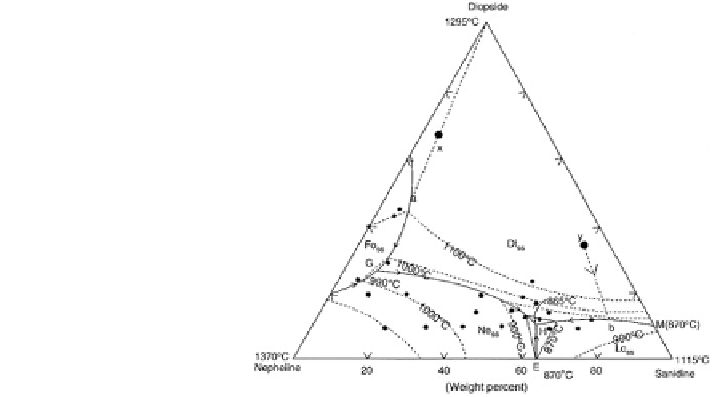
Fig. 9.2 Phase relation
in the system
diopside
-
nepheline
-
sanidine
at variable temperatures under
0.1 GPa in presence of water
(after Chattopadhyay et al.
1999)
diopside
nepheline
sanidine is, therefore, a part of the system Na
2
O
K
2
O
CaO
-
-
-
-
-
Al
2
O
3
-
SiO
2
. If the small amount of Al
2
O
3
in diopside is ignored, the system
may be described as a quinary join of the system NaAlSiO
4
-
MgO
-
KAlSiO
4
-
CaO
-
MgO
SiO
2
. Appearance of leucite in this join is related to the incongruent melting of
sanidine (Schairer and Bowen 1938; Tuttle and Bowen 1958). As pointed out earlier,
in the join diopside
-
nepheline, the appearance of forsterite
ss
and melilite is related to a
reaction relationship between diopside and nepheline. These phases were also
observed in a mixture (Di
40
Ne
60
) crystallized at 950
-
C and 0.1 GPa in the presence of
excess water. It may be observed that in the mixture Di
45
Ne
50
San
5
, crystallized at
900
°
C and 0.1 GPa [P(H
2
O) = P(Total)], forsterite and diopside co-precipitate, but
there is no melilite. At lower temperature under similar pressure, although melilite
appears in the diopside-nepheline join, there is no melilite within the diopside
°
-
nepheline
C at 0.1, 1 and
2 GPa [P(H
2
O) = P(Total)]. This is primarily because addition of sanidine causes
increase in silica activity (Carmichael et al. 1970). Thermodynamic calculation of
Carmichael et al. predicts that in a silica activity versus temperature diagram, silica
combines with melilite to form pyroxene and wollastonite before leucite reacts with
silica to form sanidine. A small amount of wollastonite supposed to appear by
desilication of melilite is possibly incorporated in the pyroxene structure as solid
solution, and thus it is not observed.
Reference to Fig.
9.2
suggests that the four-phase assemblage of Di
ss
+Ne
ss
+
Lc
ss
+ L (H) occurs at Di
11
Ne
31
San
58
and 865 + 3
sanidine system at temperatures between 550 and 600
°
-
C, whereas that of
Fo
ss
+Di
ss
+Ne
ss
+ L(G) appears at Di
26
Ne
66
San
8
and 990 + 10
°
C. The composition
of point A, where diopside
ss
+ leucite
ss
+ liquid coexist in equilibrium, was estimated
fromYoder andUpton (1971), whereas that of E, where nepheline
ss
+ leucite
ss
+ liquid
are in equilibrium (San
64
Ne
36
, 870
°
°
C), was obtained from Hamilton and MacKenzie
Search WWH ::

Custom Search