Geology Reference
In-Depth Information
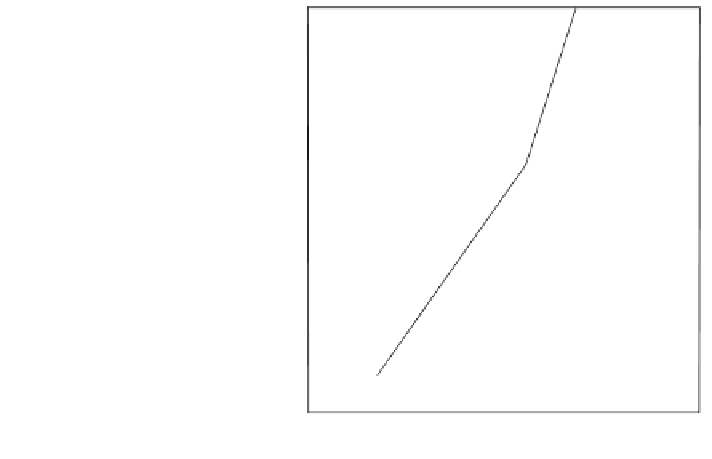
Fig. 6.5 Akermanite
-
CO
2
system under variable P-T
-
T
conditions (modi
ed after
Yoder 1973)
1.0
Mer
+
L
Di +
L + G
0.9
0.8
0.7
diopside +
calcite
L+G
0.6
0.5
0.4
0.3
akermanite+
diopside+G
0.2
0.1
800
900
1000
1100
1200
1300
1400
1500
1600
°
Temperature ( C)
Between 750
C and 1.3 GPa akermanite breaks down
to diopside + merwinite + vapour. In presence of forsterite, the akermanite stability
is further restricted. Breakdown of akermanite under CO
2
pressure (Fig.
6.5
) has
also been studied by Yoder (1973).
Willemse and Bensch (1964) provided
°
C and 0.63 GPa, and 1,050
°
field evidence of the low-temperature and
low pressure breakdown of akermanitic melilite from the Bushveld Complex. In
this area they found graphic intergrowth of monticellite and wollastonite, indicating
breakdown of akermanite. Field evidence of the intergrowth of kalsilite and
K-feldspar is also reported from the same area, along with an unidenti
ed phase,
probably leucite, which may indicate that these two phases represent the low-
temperature and low-pressure breakdown of leucite.
The above discussion suggests that leucite, along with forsterite and akermanite,
must be restricted to conditions of high temperatures and low pressure. Leucite-
bearing rocks are thus, products of volcanic and subvolcanic activities.
6.1.3 Appearance of Melilite in the Join Diopside
-
Nepheline
In natural leucite-bearing rocks, melilite, whenever present, is found to be a solid
solution of akermanite and soda-melilite.
Bowen (1922) and later Schairer et al. (1962, Fig.
6.6
) studied the join under one
atmospheric pressure, and observed the presence of a large primary phase
field of
forsterite in addition to diopside and carnegieite. Melilite appears as an important
subliquidus as well as a subsolidus phase in this join.

















































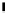









Search WWH ::

Custom Search