Geology Reference
In-Depth Information
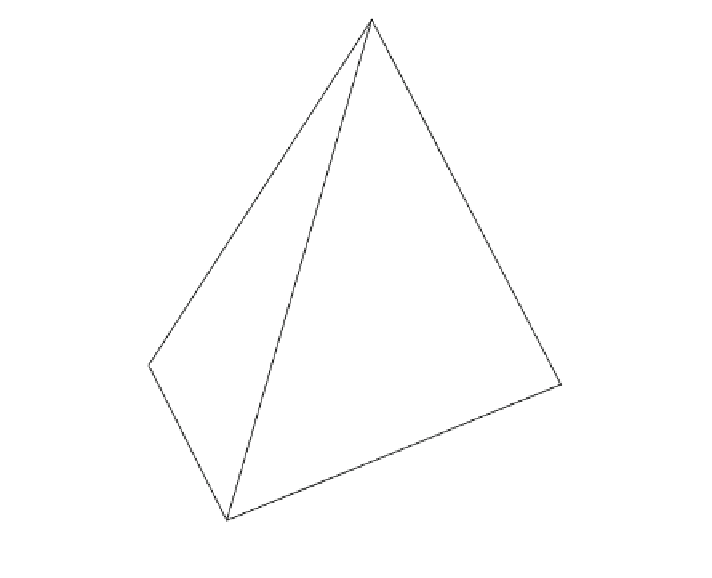
La
Wo
Ak
Mo
Dl
Ks
Lc
Sa
Qz
En
Phl
Fo
Fig. 3.9 The larnite
kalsilite
forsterite
SiO
2
tetrahedron (after Yoder 1986)
-
-
-
Wendlandt and Eggler 1980a, b, c; Gupta and Green 1988). If the bulk composition
of an initial liquid lies in this system, then under P(H
2
O) = P(Total) condition, it
should yield assemblages similar to many lamproites.
As such, the bulk composition of various K-rich rocks can be plotted in the
larnite
SiO
2
tetrahedron (Fig.
3.9
). The subtetrahedra formed in
Fig.
3.9
, was isolated by Yoder in Fig.
3.10
for easy visualization. In Fig.
3.10
,he
plotted the mineralogical compositions of many lamproites.
Yoder (1986, 1989, 1990) pointed out that the assemblage, shown as a product
of reaction (1), takes place under certain P-T
forsterite
kalsilite
-
-
-
-
T conditions [P(H
2
O) = P(Total)]:
2 Phlogopite
þ
K-feldspar
3 olivine
þ
3 leucite
þ
2H
2
O
ð
3
:
1
Þ
Two new subtetrahedra are formed because of this heteromorphic reaction, as
shown in Fig.
3.11
. Thus, the assemblage related to jumillite and shonkinite has
heteromorphic relation to absarokite and missourite, bulk compositions of which
are shown in subtetrahedra (Fig.
3.11
). Kazanite (Lacroix 1926) has similar
assemblage as missourite but the former has more modal phlogopite. Some of the
Australian lamproites such as cedricite and manilite (leucite + clinopyroxene + oliv-
ine and phlogopite) may be included with missourite, if amphibole in each is
neglected (Yoder 1986).







Search WWH ::

Custom Search