Environmental Engineering Reference
In-Depth Information
+
-
+
-
FIGURE 7.26
Structure of carbon monoxide.
C
O
C
O
C
O
burning stoves, and fireplaces. Carbon monoxide is also created as a by-product of sev-
eral industrial processes, including metal melting and chemical synthesis (USDHH
2009). Figure 7.26 shows the structure of carbon monoxide represented in three different
ways.
Carbon monoxide is one of the most common causes of fatal air poisoning (USDHH
2009). When inhaled, carbon monoxide combines with hemoglobin to produce carboxy-
hemoglobin. A condition known as anoxemia arises because carboxyhemoglobin cannot
deliver oxygen to body tissues efficiently. Exposure to high levels of carbon monoxide
may cause headache, nausea, vomiting, dizziness, lethargy, seizures, coma, and even
death (USDHH 2009). Concentrations of less than 700 ppm of CO may cause up to 50% of
the body's hemoglobin to convert to carboxyhemoglobin. In the United States, OSHA has
established a long-term exposure standard for carbon monoxide at 50 ppm.
7.8.10 Ozone
Ozone
(O
3
) is a gas present in Earth's upper atmosphere and at ground level. The ozone
occurring at ground level is considered an air pollutant by USEPA (2009h); whereas the
ozone occurring in the upper atmosphere is not considered a pollutant. In the stratosphere,
ozone is beneficial to life on Earth because it absorbs the sun's ultraviolet (UV) radiation
(USEPA 2009h). Nearer to the surface, however, the story is different. Ozone from the
ground level to a height of approximately 10 km (6 mi) in the troposphere is the main con-
stituent of urban smog (USEPA 2009h). Figure 7.27 shows the two structural forms of ozone
(USEPA 2009h).
Ozone in the stratosphere is gradually being destroyed by anthropogenically produced
chemicals referred to as ozone-depleting substances (ODS), including the following:
• Chlorofluorocarbons (CFCs)
• Hydrochlorofluorocarbons (HCFCs)
• Halons
• Methyl bromide
• Carbon tetrachloride
• Methyl chloroform
Figure 7.28 shows the two locations in the atmosphere where ozone occurs.
These substances were, and still are used in coolants, foaming agents, fire extinguish-
ers, solvents, pesticides, and aerosol propellants. Once released into the environment,
they tend to degrade very slowly (USEPA 2009h). It should also be noted that HFCs have
been substituted widely for CFCs. While this substitution has slowed the harmful effects
+
+
O
O
FIGURE 7.27
Basic molecular structure of ozone.
-
-
O
O
O
O



















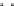




Search WWH ::

Custom Search