Biology Reference
In-Depth Information
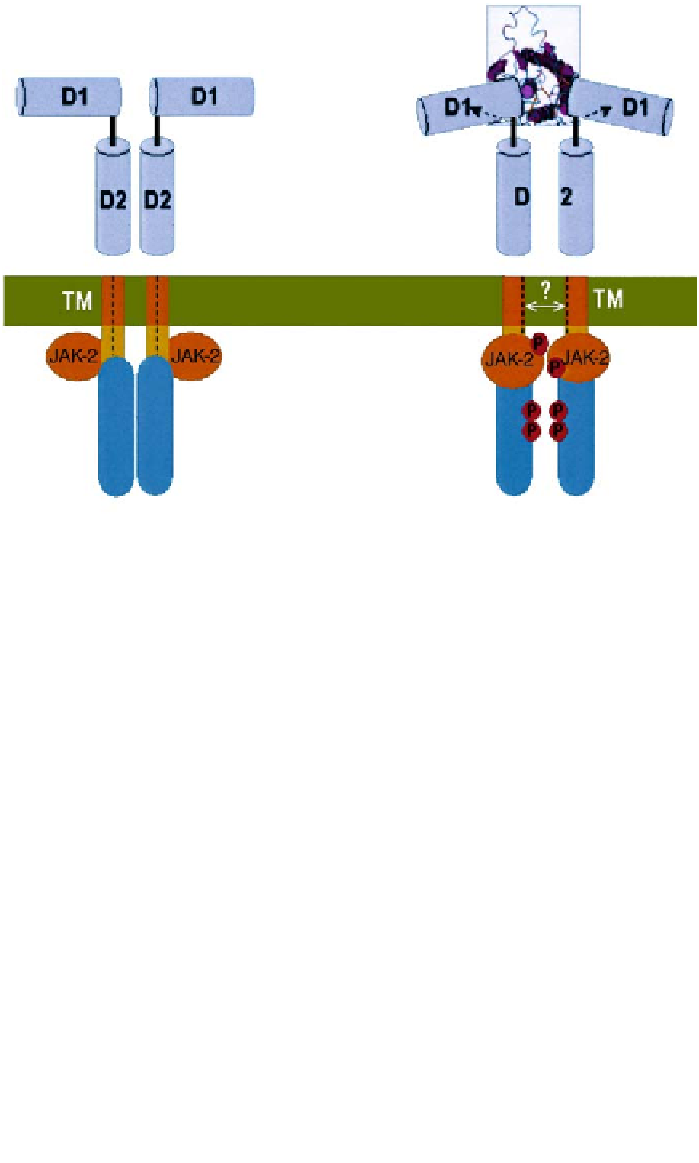
Figure 2. The unliganded EPOR exists as a symmetric dimer; dimerization is mediated mainly by the
transmembrane (TM) domains. The associated JAK-2 kinases are apart when EPOR is in its inactive
form. The dotted lines represent the middle of the TM domains in the inactive state (A). Binding of
an asymmetric EPO molecule causes a rotation of the extracellular domains, and the two D1 domains
adopt a 120° angular orientation. Coupled by the TM domain, this rotation causes similar movements
in the juxtamembrane (JM) part of the intracellular domains, thus brings the associated JAK-2 kinas-
es to a close functional organization resulting in JAK-2 activation and EPOR phosphorylation (B).
The structure in the boxed area is determined by crystallography [20]. It is not clear (as indicated by
the question mark) whether the transmembrane domains move further away from each other in the
activated state.
Orientation of EPOR dimer
The fact that EPOR in the unliganded state exists as a dimer strongly indicates
that receptor activation is achieved by a distinct conformational change in
response to EPO. An
in vivo
fragment complementation assay shows dramatic
ligand-induced enhancement of proximity of the cytoplasmic domains of EPOR
dimers [23]. Furthermore, results from EPO mimetic peptides showed that the
relative orientation of EPOR extracellular domains in a receptor dimer is direct-
ly related to the efficiency of signaling through the cytoplasmic domain [26].
For example, the EPO-mimetic peptide EMP dimerizes EPOR with two-fold
symmetry and triggers activation of EPOR signaling cascades. A derivative of
EMP, EMP33, acts as an antagonist of EPO. The inactive complex of two EPOR
extracellular domains with EMP33 differs from that of the active complex
formed with EMP by a 15º deviation from the axis of symmetry. The difference