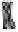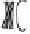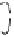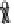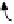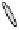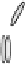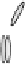Biology Reference
In-Depth Information
compounds active in the assay may activate EPOR by a different mechanism
than by rHuEPO. Another strategy is to identify molecules that directly bind
to and agonize EPOR in a manner similar to that of rHuEPO. The latter strat-
egy can be performed in two steps: the first step is to identify compounds that
bind EPOR and the second step is to covalently link the compounds into biva-
lent dimers that can agonize the receptor by EPOR homodimerization.
The latter strategy takes advantage of the observation that an EPOR mutant
containing an Arg129 to Cys129 mutation was constitutively active [52]. A
disulfide bond formed between the Cys129 residues on the receptors resulted in
homodimerization and receptor activation demonstrating that EPO was not
essential for receptor activation (Fig. 2). X-ray crystallography results demon-
strated that EPOR forms a 2:1 complex with EPO [57]. Each receptor uses the
same region on its surface to bind to two surfaces on EPO, resulting in recep-
tor homodimerization. Further confirmation of the homodimerization mecha-
nism was the discovery of agonist monoclonal IgG antibodies that could
homodimerize EPOR [53]. Anti-EPOR antibodies activated because they were
bivalent, had two binding sites, and could simultaneously bind and cross-link
two EPOR (Fig. 2). Monovalent, Fab fragments could bind but did not agonize
EPO
mimetic
peptide
Small
molecule
mimetic
Agonist
Anti
b
ody
EPO
EPO dimer
Compound 5
membrane
P
JAK2
JAK2
P
P
JAK2
JAK2
P
P
JAK2
JAK2
P
P
JAK2
JAK2
P
P
JAK2
JAK2
P
P
JAK2
JAK2
P
HC
P
P
P
HCP
HC
P
P
P
HCP
HC
P
P
P
HCP
HC
P
P
P
HCP
HC
P
P
P
HCP
HC
P
P
P
HCP
Signal
transduction
HCP action
terminates
signal
Figure 2. Mechanism of erythropoietin receptor (EPOR) activation. EPOR are homodimerized
because of the two asymmetric receptor-binding sites on rHuEPO. EPO binding results in phospho-
rylation of EPOR, JAK-2 recruitment, and phosphorylation of JAK-2. The activation of JAK-2 results
in downstream signaling events. Hematopoietic cell phosphatase (HCP) can bind the activated (phos-
phorylated) receptor resulting in dephosphorylation of JAK-2, thereby terminating signal transduc-
tion. The EPO mimetic compounds; agonist antibody [53], EPO dimer [46, 47], EPO mimetic peptide
[54], compound 5 [55], and small-molecule mimetics [55, 56] can all homodimerize and activate
EPOR in a manner similar to that of rHuEPO.