Biology Reference
In-Depth Information
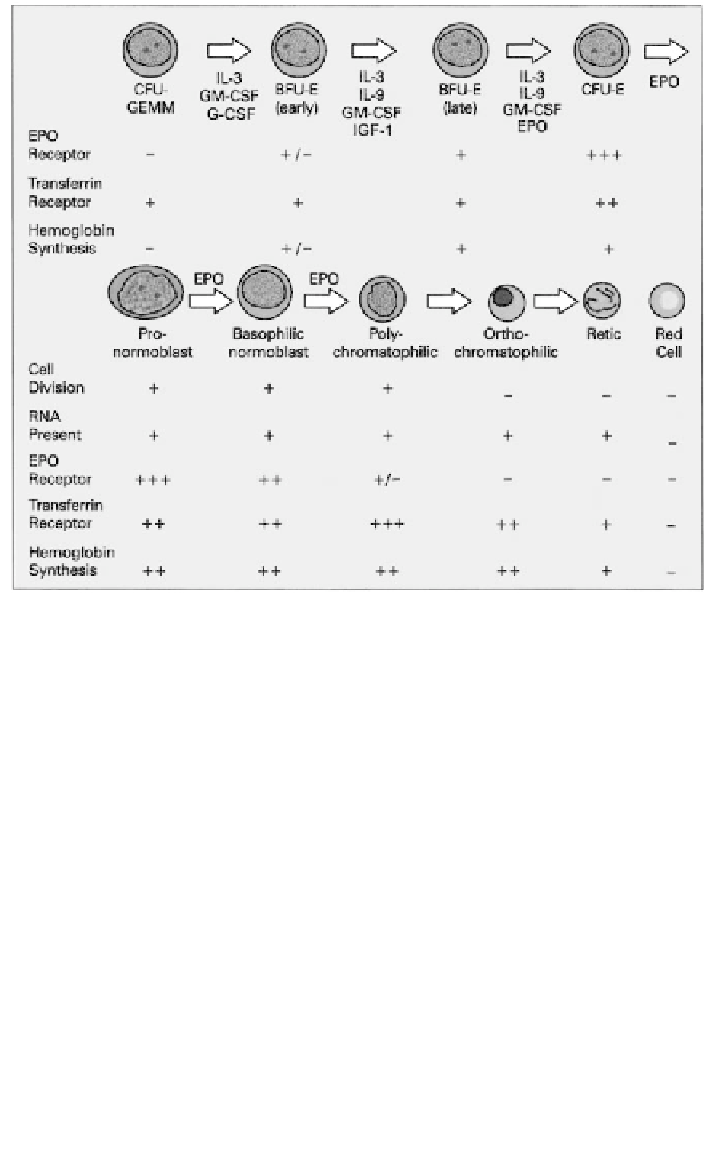
Figure
1. Erythropoiesis,
from CFU-GEMM to erythrocyte; developmental stages,
regulatory
cytokines, cell-surface receptors, and hemoglobin synthesis.
Transferrin receptors (TfR) are common to most mammalian cells, provid-
ing access for transferrin-bound iron required for the synthesis of heme and
other iron-containing proteins. Many TfR are present on hemoglobin-synthe-
sizing cells, reaching a peak of some 800,000 per cell on polychromatophilic
normoblasts, and decreasing to 100,000 on reticulocytes. TfR are absent from
mature red cells: as hemoglobin synthesis has ceased, no further iron is
required. TfR preferentially bind diferric transferrin, although monoferric also
can be bound. With TfR binding of the iron-bearing transferrin, the complex is
endocytosed and the iron is released into the cytoplasm. The receptor-trans-
ferrin complex then returns to the membrane where the transferrin is released
into the plasma and the receptor again becomes available [10]. Hypoxia-
induced upregulation of transferrin synthesis is controlled by the transcription
factor hypoxia-inducible factor-1 (HIF-1) [11] (see below).
The earliest microscopically identifiable erythrocyte precursor in bone mar-
row aspirates is the normoblast, 15 to 20 µm in diameter with a large nucleus
containing multiple nucleoli and basophilic cytoplasm. Further cell division
results in the appearance of basophilic and then polychromatophilic nor-