Biology Reference
In-Depth Information
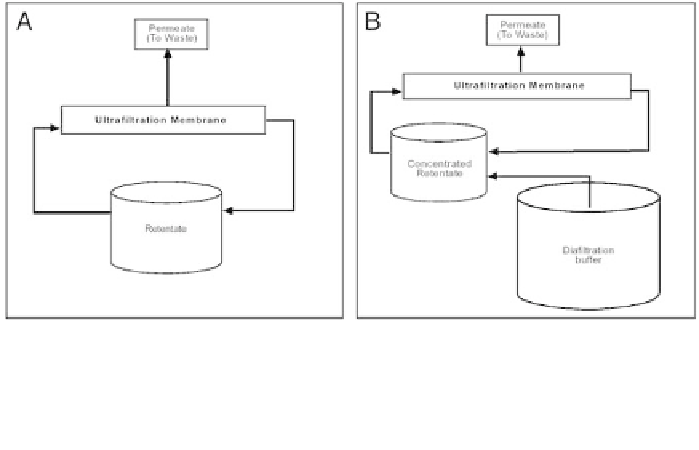
Figure 10. Panel A - Concentration mode. Clarified cell culture medium volume is reduced in reten-
tate to make concentrated retentate. Panel B - Diafiltration (DFM) mode. Removal of high-conduc-
tivity components in concentrated retentate to make DFM.
media retentate at approximately the same rate as the permeate flux rate
(Fig. 10b). This process causes a buffer exchange of the original high conduc-
tivity buffer for the lower one. The greater the number of retentate volumes
pumped though the membrane during the diafiltration step, the more complete
the buffer exchange. This product pool is referred to as diafiltered media,
which either can be immediately processed through the subsequent purifica-
tion steps or stored frozen and processed later.
Chromatography: isoform selection
An anion-exchange media can be used to separate molecules on the basis of
molecular charge. This chromatography may be used to separate rHuEPO
from host cell proteins, nucleic acids, and endotoxin.
Chromatography: removal of contaminant proteins
A reversed-phase chromatography step can be used to remove host cell pro-
teins and additional nucleic acids. The interactions of the column matrix with
proteins are mostly hydrophobic in nature. Once bound to the column, a pro-
tein can be eluted with another hydrophobic, low-dielectric organic solvent.
The protein portion of rHuEPO is quite hydrophobic because of the large pro-
portion of hydrophobic residues and its solubility characteristics after removal
of the polysaccharide portion of the molecule. Gel filtration chromatography,
also known as size-exclusion chromatography or molecular sieving, may also
be used to separate a protein mixture based on molecular weight. The media