Information Technology Reference
In-Depth Information
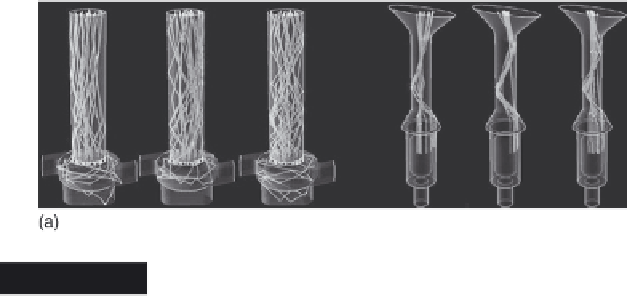
Carrier particle trajectory inside the inhaler at 60 L/min
(from left, d
particle
= 32, 108, and 275 μm): (a) Aerolizer®,
(b) Handihaler® (reprinted from Donovan et al., 2012;
with permission from John Wiley & Sons)
Figure 7.8
and particles are accelerated and directed towards the inhaler wall and
then towards the inhaler exit, without any swirling motion. It was
observed that the number of particle-inhaler collisions is more dependent
on carrier particle size in the case of the Aerolizer
®
, than in case of the
Handihaler
®
, with a greater number of collisions when larger carrier
particles were used. This was attributed to the presence of the swirling
motion and longer residence time inside the mouthpiece of the Aerolizer
®
.
Furthermore, the performance of the Aerolizer
®
was infl uenced by carrier
particle morphology, while performance of the Handihaler
®
was relatively
independent of surface roughness. Coupling the CFD simulations with
in
vitro
results, the authors concluded that impaction-based forces are not
the dominant mechanism in drug detachment from carrier particles in the
Handihaler
®
, in contrast to the Aerolizer
®
, and therefore both physical
properties of the carrier and the predominant detachment mechanism
have to be taken into account when analyzing DPI performance.
7.3.2 Dissolution apparatus hydrodynamics
Since the 1960s and 1970s, when the importance of dissolution tests in
drug quality control assessment was recognized and extensive work was
done on development and standardization of dissolution apparatus, until
nowadays dissolution testing has become an indispensable tool for
quality control of various dosage forms, and the fi eld of its possible
applications has been considerably expanded (Dressman and Krämer,
2005). Dissolution testing is widely used in the pharmaceutical industry
for optimization of formulation, testing of batch-to-batch reproducibility,





Search WWH ::

Custom Search