Chemistry Reference
In-Depth Information
Fe
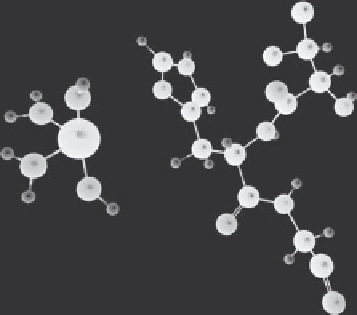
FIGURE 2.6 (See color insert.)
Pictorial.representation.of.the.Fe
3+
.ion.coordination.to.the.
His50. residue. of. the. wild-type. α-synuclein. protein. in. water. at. room. temperature. obtained.
from.QM/MM.simulations.
due. to. the. presence. of. Asp119,. Asp121,. Tyr125,. Tyr133,. Asp135,. and. Tyr136,. or.
.simply.the.presence.of.all.these.Asp.and.Tyr.residues.might.be.suficient.to.attract.
metal ions.
We.report.results.of.a.study.of.the.binding.of.Fe(III).with.the.His50.residue.of.
the.wild-type.and.mutant.α-synuclein.proteins.in.water..The.initial.simulation.starts.
with.the.Fe
3+
.in.an.octahedral.Fe(OH)
3
(H
2
O)
3
.structure.(taken.from.previous.studies).
at.a.distance.of.11.Å.from.the.His50.residue.of.both.proteins..We.ind.that.the.Fe
3+
.
ion.coordinates.to.the.imidazole.ring.N.atoms.of.the.His50.residue.within.12.and.
19.ps.for.the.A53T.and.wild-type.α-synuclein.proteins.in.water,.respectively..This.
result.supports.previous.experimental.measurements..For.the.wild-type.α-synuclein.
in.aqueous.solution,.we.ind.that.the.Fe
3+
.ion.coordinates.to.two.hydroxyl.groups.and.
two. water. molecules. and. to. the. two. imidazole. N. atoms. with. average. distances. of.
3.6.±.0.5.and.4.8.±.0.3.Å,.respectively.(see.Figure.2.6).
For.the.A53T.protein,.our.results.suggest.a.totally.different.coordination.mecha-
nism.from.that.of.the.wild-type.α-synuclein..The.Fe
3+
.ion.coordinates.to.one.imid-
azole.ring.N.atom,.one.hydroxyl.group,.and.one.water.molecule..One.imidazole.ring.
N. atom. loses. its. hydrogen. atom. via. hydrogen. bonding. interactions. with. the. free.
hydroxyl. group. in. water.. The. distance. between. the. Fe
3+
. ion. and. the. coordinating.
imidazole.N.atom.is.shorter.with.an.average.distance.of.2.9.±.0.3.Å.in..comparison.to.
the.distance.obtained.for.the.Fe
3+
.ion.coordination.with.the.wild-type.α-synuclein.
His50.residue.(see.above)..The.Fe
3+
.ion.also.coordinates.to.the.peptide.N.atom.located.
between.the.Val49.and.His50.residues.with.a.distance.of.4.7.±.0.4.Å.(see.Figure.2.7)..
Our.indings.indicate.that.the.Fe
3+
.coordination.mechanism.and.the.structures.and.
conformations. obtained. for. the. resulting. metalloproteins. are. different. from. each.
other. for. the. wild-type. and. A53T. mutant-type. α-synuclein. proteins. in. water.. We.
also.note.that.the.water.chemistry.around.these.peptides.is.notably.different.for.the.
mutant. and. wild-type. α-synuclein. proteins.. These. differences. might. be. attributed.
to.the.different.folding.and.thermodynamic.preference.of.these.two.proteins,.which.
were.described.in.detail.earlier.
Search WWH ::

Custom Search