Chemistry Reference
In-Depth Information
(a)
A
B
C
D
E
F
(b)
A
B
C
D
E
F
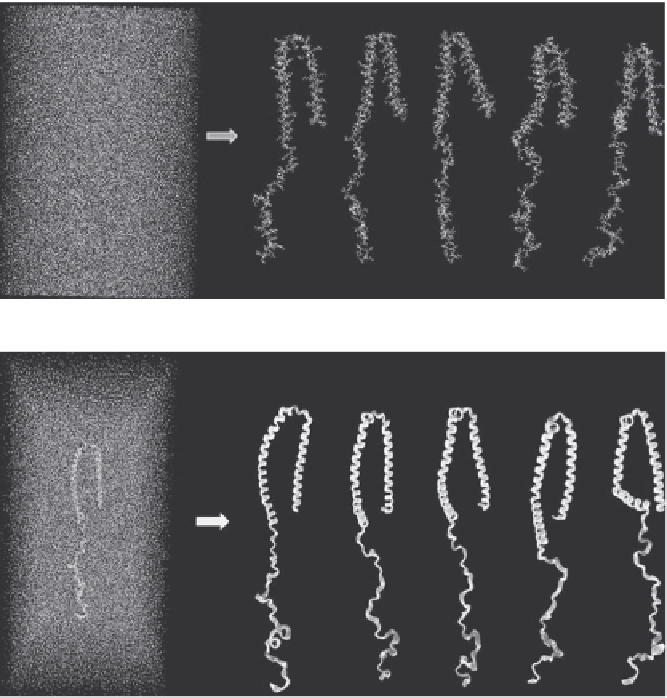
FIGURE 2.4
(a). Structures. of. A53T. α-synuclein. in. aqueous. solution. (A). obtained. from.
classical.MD.simulations.at.(B).0.ns,.(C).1.ns,.(D).2.ns,.(E).3.ns,.and.(F).4.ns..(b).(
See color
insert.
). Secondary. structures. of. A53T. α-synuclein. in. aqueous. solution. (A). obtained. from.
classical. MD. simulations. at. (B). 0.ns,. (C). 1.ns,. (D). 2.ns,. (E). 3.ns,. and. (F). 4.ns.. The. α-helix.
(purple),.3
10
.helix.(orange),.bridge.β.(blue),.turn.(green),.and.coil.(red).are.depicted.
solvated. structures. are. more. pronounced. when. looking. at. the. secondary. structure.
(Figures. 2.4b. and. 2.2b).. The. wild-type. structure. does. not. exhibit. any. interaction.
between.the.N-terminus.and.the.rest.of.the.protein.in.aqueous.solution,.but.in.the.
case. of. the. A53T. mutant,. there. does. appear. to. be. some. interaction. between. the.
N-terminus.and.the.C-terminal.α-helix..The.interaction.is.not.as.pronounced.as.in.
the.gas.phase.and.is.partially.inhibited.by.the.presence.of.water.molecules,.but.not.
as. completely. inhibited. as. the. wild-type. in. aqueous. solution.. The. C-terminal. tail.
is. again. most. lexible. in. the. solvated. molecular. dynamic. simulations.. The. region.
between. residues. 68. and. 71. undergoes. signiicant. structural. changes. during. the.
course.of.the.simulation.as.well..The.turn.and.coil.section.between.the.two.α-helical.
Search WWH ::

Custom Search