Chemistry Reference
In-Depth Information
20
A
C
D
E
15
10
ξ
5
0
10
12
14
16
(a)
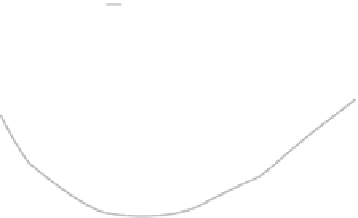
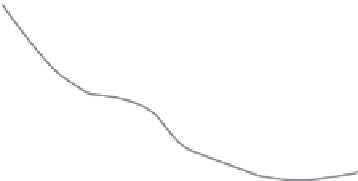
(b)
Reaction coordinate ξ (Å)
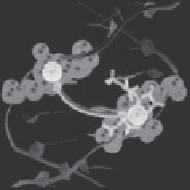
FIGURE 1.5 (See color insert.)
(a).Backbone.atoms.and.their.centers.of.mass.(transpar-
ent.and.solid.blue.spheres,.respectively).deining.the.reaction.coordinate.ξ..(b).Free-energy.
proiles.(kcal/mol).for.
A
-
E
..Small.arrows.indicate.the.free-energy.minima,.and.the.yellow.
bar.shows.the.minor.groove.width.of.DNA-HMG.
opening.the.minor.groove.incurs.a.high.free-energy.cost.(Δ
G
.~.7-8.kcal.mol
−1
).for.
A
.and.
B
.which.substantially.increases.for.
C
.(Δ
G
.~.15.kcal.mol
−1
),.which.is.higher.
for.dinuclear.drugs.than.for.canonical.B.DNA.(Δ
G
.~.5.kcal.mol
−1
).and.for.the.cor-
responding.cisplatin-DNA.adduct.(Δ
G
.~.0.kcal.mol
−1
).
126
Thus,. the. formation. of. diplatinum. drug-DNA. adducts. may. signiicantly. affect.
the.binding.of.excision.repair.enzymes.and.other.proteins.involved.in.the.cytotoxic.
activity.of.common.Pt.drugs..Subsequently,.this.process.could.lead.to.a.different.cel-
lular.response.and,.in.turn,.to.a.lower.resistance.and.cross-resistance.with.respect.to.
cisplatin.
126
.Although.the.development.of.drug.resistance.is.a.highly.complex.mecha-
nism,.our.indings.provide.an.additional.rationale.for.the.improved.cytotoxic.activity.
of.these.compounds.in.cell.lines.resistant.to.cisplatin.
1.3.3 c
atalytic
m
echaniSm
of
m
etallo
β
-l
actamaSeS
β-Lactams.are.the.most.widespread.antibiotics.on.the.market..They.have.exerted.a.
large.evolutionary.pressure.on.bacteria,.triggering.sophisticated.resistance.mecha-
nisms.. Among. them,. the. most. used. is. the. expression. of. β-lactamases,. hydrolases.
which. use. different. protein. scaffolds. and. catalytic. architectures. to. inactivate.
β-lactam.drugs.
156
β-Lactamases.from.classes.A,.C,.and.D.are.the.serine.hydrolases,.whereas.class.
B.β-lactamases.are.characterized.by.the.presence.of.Zn.ions.bound.to.their.active.
sites.
157
. Metallo. β-lactamases. (MβLs),. despite. not. being. as. ubiquitous. as. serine.
β-lactamases,.hydrolyze.all.kinds.of.β-lactam.antibiotics,.including.the.latest.gener-
ation.of.carbapenems.(Scheme.1.1)..MβLs.are.increasingly.spreading.among.patho-
genic.bacteria.in.the.clinical.setting.and.are.resistant.to.all.current.clinical.inhibitors.
on.the.market.
158-161
.Thus,.understanding.their.function.at.the.molecular.level.is.of.
paramount.importance.for.designing.effective.drugs.













Search WWH ::

Custom Search