Chemistry Reference
In-Depth Information
given.in.Ref..[192]..The.static.mean.polarizabilities.and.the.polarizability.anisotro-
pies.of.copper.clusters.up.to.the.nonamer.were.calculated.with.the.PB86.functional.
at.the.optimized.geometries.obtained.by.using.the.VWN.functional.[192]..In.Figure.
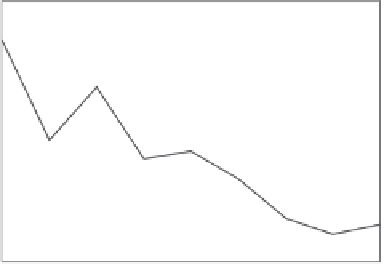
9.23,.the.mean.polarizability.per.atom.of.copper.clusters.is.plotted.
As.this.igure.shows,.going.from.the.atom.up.to.the.pentamer,.the.mean.polariz-
ability.per.atom.has.an.oscillating.behavior..From.the.pentamer,.the.polarizability.
per.atom.decreases.with.a.minimum.value.for.the.octamer..It.increases.then.again,.
going.from.the.octamer.to.the.nonamer.
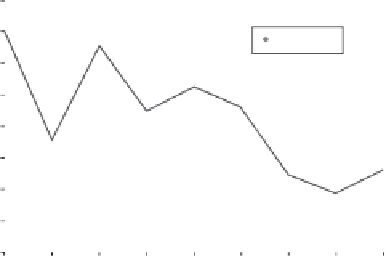
In.Figures.9.24.and.9.25,.the.experimental.polarizability.per.atom.of.sodium.and.
lithium.clusters.up.to.the.nonamer.is.plotted,.respectively.
50
Cu-(B88-P86)
40
30
1
2
3
4 5
Number of atoms
6
7
8
9
FIGURE 9.23
Calculated.mean.polarizability.per.atom.of.Cu
n
.(
n
.=.2-9)..The.calculations.
were.performed.using.the.B88-P86.functional..(Reprinted.from.Calaminici,.P..et.al.,.
J. Chem.
Phys
.,.113(6),.August.8,.2199,.2000..With.permission.)
170
160
150
140
130
120
110
100
90
1
Na-Exp.
2
3
4 5
Number of atoms
6
7
8
9
FIGURE 9.24
Experimental.mean.polarizability.per.atom.of.Na
n
.(
n
.=.2-9)..Values.are.from.
Ref..[188]..(Reprinted.from.Calaminici,.P..et.al.,.
J. Chem. Phys
.,.113(6),.August.8,..2199,.2000..
With.permission.)


















Search WWH ::

Custom Search