Chemistry Reference
In-Depth Information
i-Pr
N
N
N
N
TPIH
HIPT
i-Pr
Mo
HIPT=
i-Pr
N
TPIH
N
Mo
i-Pr
N
N
Na
i-Pr
i-Pr
(A)
(B)
(C)
NH
3
N
2
Mo
1
Mo-NH
3
Mo-N
2
2
+c
-
14
+H
+
Mo-NH
3
1+
Mo-NNH
1+
13
3
+H
+
+
NH
Mo-NH
2
NH
2
TPIH
TPIH
TPIH
HIPT
12
N
HIPT
+e
-
N
N
N
N
Mo
TPIH
N
Mo
Mo-NH
2
1+
N
N
+H
+
N
N
11
+H
+
Step II
Mo-NH
Mo-NNH
2
Step I
6
+H
+
TPIH
+
TPIH
Mo-NNH
2
HIPT
N
HIPT
NH
N
7
N
N
N
N
+e
-
N
Mo
TPIH
Mo
TPIH
N
+H
+
N
NH
3
(D)
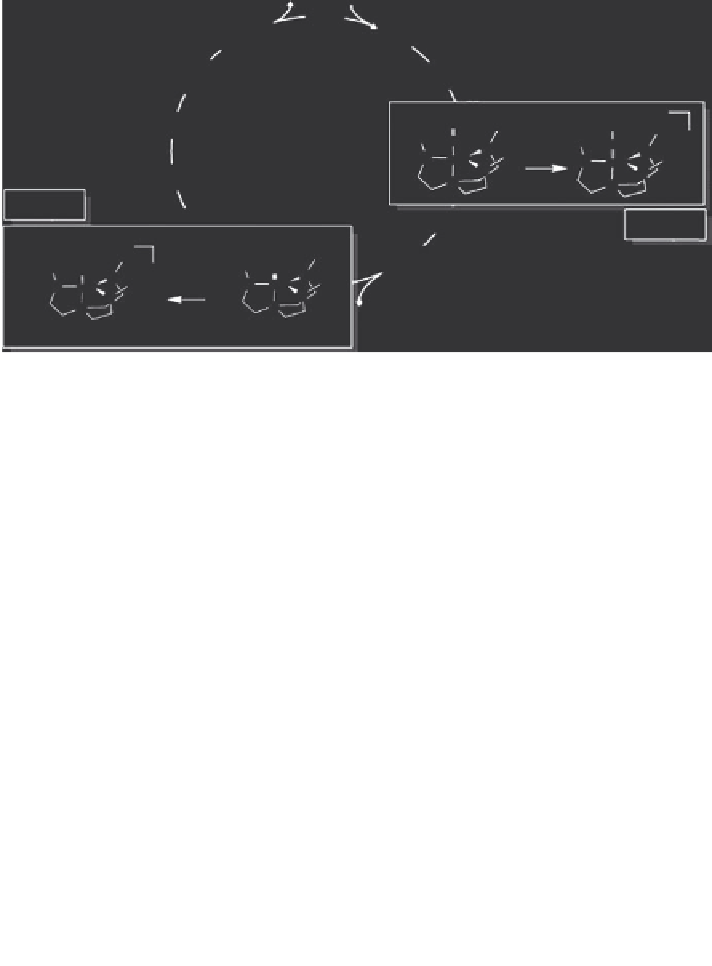
FIGURE 1.1
(A).Experimental.catalyst.and.(B).theoretical.model..(C).Proton.source,.LutH
+
..
(D).Reactions.
I
.and.
II
.from.the.putative.nitrogen.ixation.cycle..(Reprinted.with.permission.
from. Alessandra. Magistrato. et. al.,.
J. Chem. Theory Comput
.,. 3(5),. 1708.. Copyright. 2007.
American.Chemical.Society.)
TABLE 1.1
Reaction Energies in Solution (ΔE
R
, kcal/mol) of Step I and Step II:
DFT vs. Experimental Results
DFT
Expt.
C
6
H
6
C
7
H
16
C
6
H
6
B3LYP
(PCM)
a
B3LYP
(PCM)
b
BH&H
a,d
BH&H
c,d
BH&H
a,d
BH&H
c,d
Step.
I
−8.7
4.7
2.9
−10.2
−5.3
−4.7
0
Step.
II
−7.9
6.4
4.6
−13.2
−3.6
−3.0
−1
a.
With.6-31+G(d).
b.
B3LYP/TZVP,.Ref..125.
c.
With.BH&H/6-311++G(d,p).
d.
Considering.solvated.LutH
+
.and.Lut.with.four.solvent.molecules.










Search WWH ::

Custom Search