Chemistry Reference
In-Depth Information
Fe
A
4.1 Å
3.5 Å
H3
H4
O3
O4
H2
O6
O2
4.9 Å
O5
C1
Fe
B
O1
ψ
5.2 Å
C7
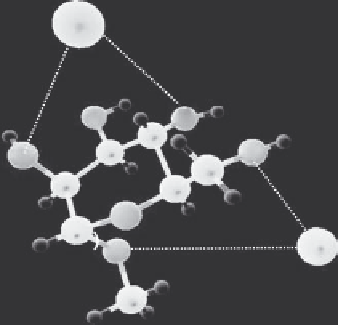
FIGURE 3.3
Methyl-α-d-mannopyranoside.and.its.complex.with.two.Fe
3+
.ions.in.the.gas.
phase,.obtained.from.CPMD.simulations.
the. hydroxyl. groups. when. using. force. ield. parameters,. i.e.,. failing. to. distinguish.
between.axial.and.equatorial.orientations.of.the.hydroxyl.groups.and.ignoring.the.
impact.of.the.conformational.changes.and.steric.effects.on.the.electrostatics.
To.investigate.the.interaction.of.M.with.more.than.one.Fe
3+
.ion.in.the.gas.phase,.
we.calculated.the.chemical.reactivity.indices.of.each.atom.in.the.vicinity.of.a.Fe
3+
.ion.
(M-Fe
3+
).and.simulated.M.with.two.Fe
3+
.ions.(Figure.3.3)..Our.chemical.reactivity.
index.calculations.using.the.condensed.FFs.indicate.that.the.second.Fe
3+
.ion.might.
be.coordinated.to.the.O2.atom.and.to.the.glycosidic.linkage.O1.atom.(Table.3.1).with.
f
HOMO
.values.of.0.50.and.0.18,.respectively..CPMD.simulations.show.that.the.distances.
between.the.second.Fe
3+
.ion.and.the.O1.and.O2.atoms.are.shorter.than.the.distance.
between.the.second.Fe
3+
.ion.and.the.rest.of.the.hydroxyl.oxygen.atoms.of.M..In.addi-
tion,.these.simulations.show.that.the.distances.between.the.second.Fe
3+
.ion.and.the.
possible.coordination.sites.of.M.are.larger.than.those.obtained.for.the.single.Fe
3+
.ion.
in.the.gas.phase..These.results.might.indicate.that.the.second.Fe
3+
.ion.coordination.is.
weaker.than.the.coordination.of.a.single.Fe
3+
.ion.to.M.in.the.gas.phase..CMD.simula-
tions.overestimate.the.mobility.of.the.Fe
3+
.ions.in.comparison.to.CPMD.simulations.
To.further.evaluate.the.binding.afinity.of.two.Fe
3+
.ions.to.M,.we.computed.the.
binding.energies.for.each.Fe
3+
.ion.utilizing.the.trajectories.taken.from.our.CPMD.
simulations.using.the.following.relationship:
A+B
→
AB
BE
=
E
−
E
−
E
.
AB
A
B
where
BE.represents.the.binding.energy
A.and.B.represent.the.Fe
3+
.ion.and.M
According.to.these.calculations,.the.coordination.of.the.single.Fe
3+
.ion.is.thermo-
dynamically. preferred. with. a. BE. value. of. -164.2.kJ. mol
−1
.. On. the. other. hand,. the.
Search WWH ::

Custom Search